TFEB and lysosome regulation and signaling in aging and AD
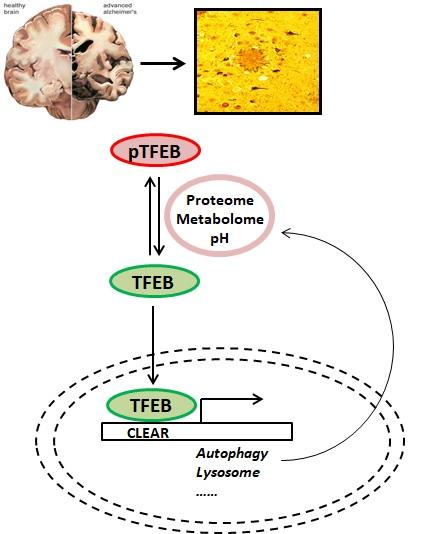
Accumulating evidence has implicated impaired autophagy-lysosome pathway in neurodegenerative diseases including Alzheimer’s disease. The Transcription Factor EB (TFEB) was discovered as a master regulator of cellular clearance through coordinated expression of autophagy and lysosomal target genes. We found that TFEB is highly efficacious in ameliorating Tau/NFT pathology and behavioral deficits in Tau transgenic mice. We seek to identify Tau-induced lysosomal changes for therapeutic targeting. This project is an integral component of a Program Project Grant (P01 AG066606) aimed at understanding how lysosomal function is regulated through lysosome-to-nucleus signaling pathways, how these pathways are changed in aging and Alzheimer’s disease, and how to harness these regulatory pathways to promote brain health, combat age-associated functional decline, and delay neurodegenerative diseases.
Role of soluble epoxide hydrolase in neuroinflammation and AD
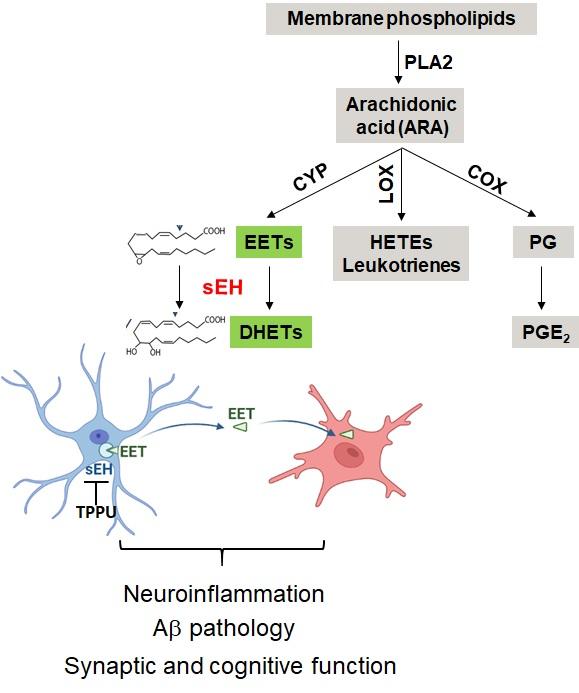
Neuroinflammation has been increasingly recognized to play a critical role in AD. The epoxy fatty acids (EpFAs) are derivatives of the arachidonic acid (ARA) metabolism with anti-inflammatory activities. However, their efficacy is limited due to the rapid hydrolysis by the soluble epoxide hydrolase (sEH). Using a specific small molecule sEH inhibitor (TPPU), we reported that long-term treatment of TPPU to the 5xFAD mice dampened neuroinflammation and reduced β-amyloid pathology. Our current research is aimed at addressing the following questions: how does the astrocytic sEH pathway communicate with microglia to regulate neuroinflammation? What is the role of brain vasculature known to subject to sEH regulation in this process? Whether and how sEH inhibition affect other ARA associated pathways and their lipid derivatives? Overall, these studies will provide critical information concerning the mechanism-of-action and therapeutic targeting of sEH. It will also afford an in-depth understanding of the ARA metabolism and identify new targets that impact AD pathogenesis.
Complement signaling in neuron-immune interaction and immunometabolism
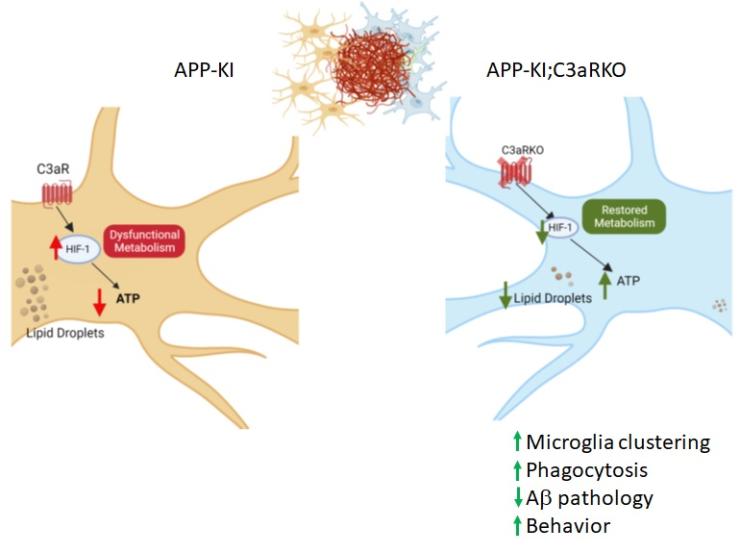
A major driver of neuroinflammation is the complement pathway, an evolutionarily conserved innate immune signaling mechanism. C3, a central component of the complement signaling, is cleaved to C3a which interacts with C3aR to elicit downstream responses. Our earlier study mapped out an astroglial C3 and microglial C3aR axis that governs network function and innate immunity in the context of AD and tauopathy and that C3aR inactivation conferred protection against age-associated functional decline and AD neuropathology. Our recent work implicates the C3-C3aR signaling in regulating cellular metabolism involving HIF-1α signaling and lipid metabolism. Building on this novel finding, we aim to perform in-depth analysis of the mechanism and function of the C3aR-HIF-1α pathway in immunometabolism and AD pathogenesis.
Uncover cell surface proteome in aging and AD
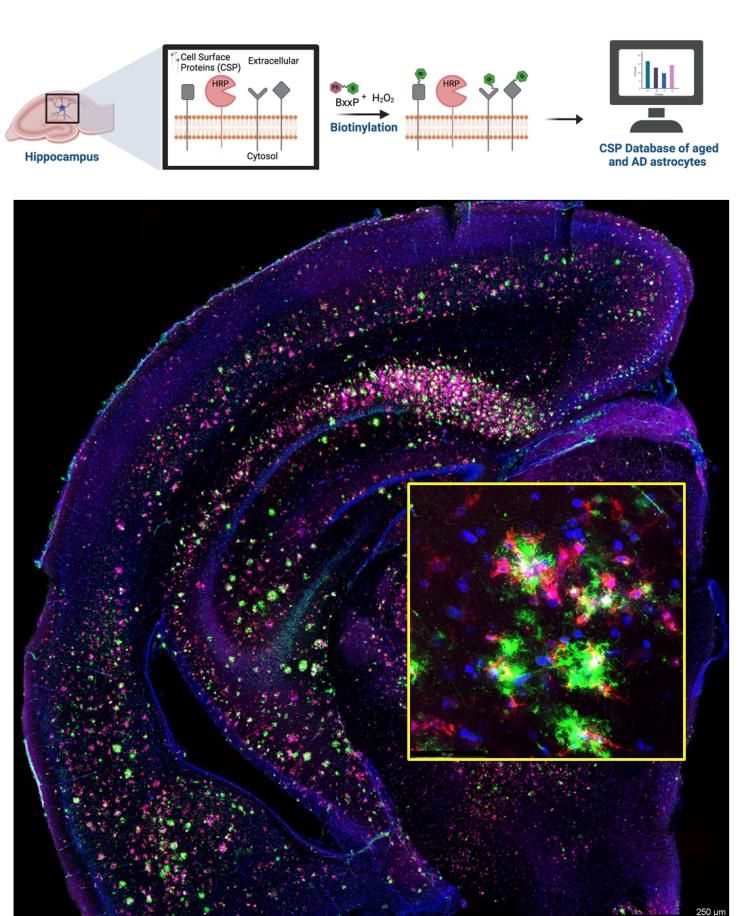
Astrocytes and microglia play essential roles in sensing brain microenvironment and maintaining neuronal function through cell-cell communications mediated by their cell surface proteins (CSPs). Reactive astrogliosis and microgliosis are prominent features of AD and these are associated with profound morphological and transcriptomic changes. How these lead to the remodeling of cell surface proteins are not understood. We aim to fill this knowledge gap to decode the CSPs in astrocytes and microglia in aging and AD mouse models. We will be using a state-of-the-art in situ cell surface proteome extraction by extracellular labeling (iPEEL) method by creating a mouse line expressing a membrane-bound horseradish peroxidase (HRP) in astrocytes and microglia and identifying the CSP landscape in aging and in response to AD pathology using ratiometric tandem mass tag mass spectrometry (TMT-MS). This will be followed by integration with astrocyte and microglia transcriptome to select candidate targets for functional testing in human iPSC-derived astrocyte and microglial cultures and in AD mouse models. This represents a new and exciting addition to our research portfolio.








