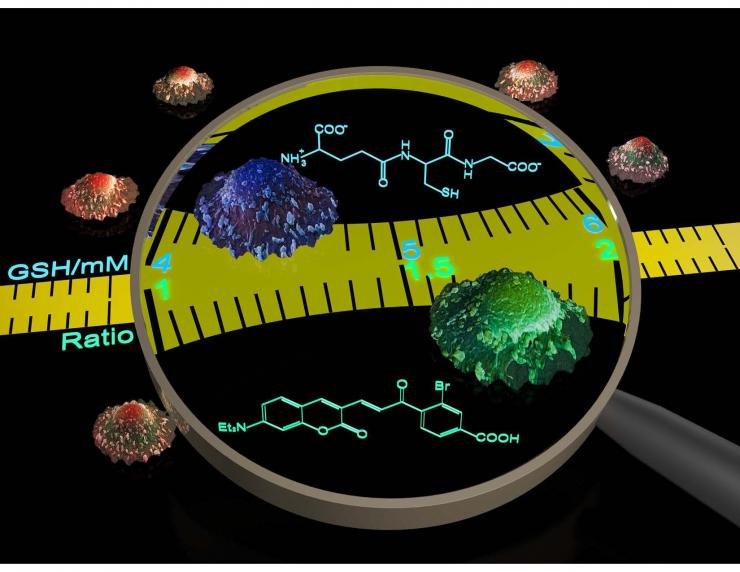
Quantitative Real-Time Imaging of GSH Dynamics
Glutathione (GSH) is the most abundant non-protein thiol in eukaryotic cells. Together with its oxidized partner (GSSG), GSH maintains cellular redox homeostasis and regulates protein functions through S-glutathionylation. These important functions are dynamically regulated by the intracellular concentration and distribution of GSH. Currently, the concentration of intracellular GSH is derived from either cell lysates or GSH-S-transferase (GST) dependent probes. These approaches, however, cannot provide information about the real-time dynamics of GSH concentration changes.
Our group reported the first reversible reaction-based GSH probe (ThiolQuant Green) that can perform single-point quantification of GSH levels in living cells (ACS Chem Bio 2015). We developed a computational model to predict the thermodynamics and kinetics of thiol-Michael addition reactions (Organic Letters 2015), which guided the development of our 2nd generation GSH probe—RealThiol—that allows real-time monitoring of GSH dynamics in living cells (Nature Communications 2017). Furthermore, we have developed a series of organelle-specific GSH probes based on RealThiol (ACS Sensors 2017Antioxidants and Redox Signaling 2018) and have shared the RealThiol probe with >50 redox biology labs around the world through either Kerafast.com or Material Transfer Agreement (MTA). We expect RealThiol will become the "gold standard" for GSH quantification in living cells.
Drug Discovery towards Coactivator Targeted Therapies
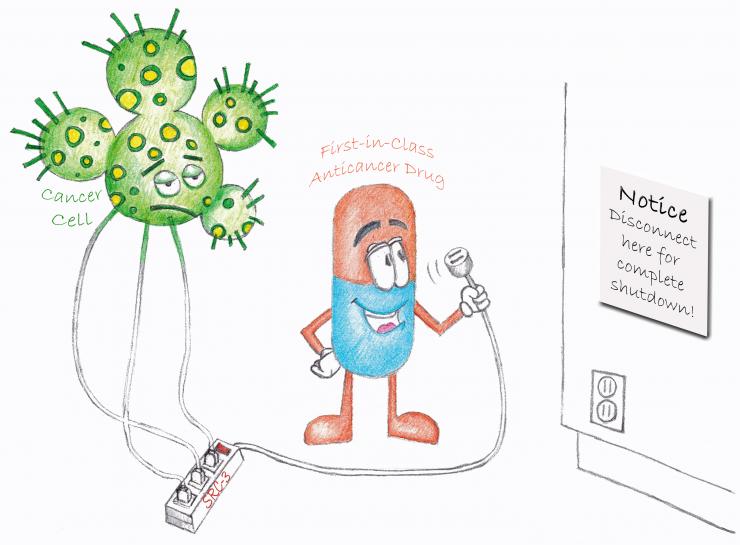
Most targeted therapeutic drugs available are designed to inhibit only one pathway. However, drug resistance often occurs when tumors shift to alternative growth pathways, rendering the originally targeted pathway inessential for tumor growth. As a master regulator of cellular growth and development, steroid receptor coactivator-3 (SRC-3) sits at the nexus of many intracellular signaling pathways critical for cancer proliferation and metastasis. Targeting SRC-3, therefore, can impact many cancer signaling pathways simultaneously and may alleviate drug resistance problem. Through high throughput screening (HTS), the O’Malley group identified bufalin as a potent proof-of-concept SRC-3 small molecule inhibitor (SMI). Our group developed bufalin nanoparticles to reduce its cardiotoxicity (Cancer Research 2014) and synthesized a bufalin prodrug to enhance the aqueous solubility (PLoS One 2015). However, due to its strong cardiotoxicity, bufalin has limited translational potential.
We re-analyzed the HTS hits for SRC-3 SMIs and identified SI-1 with an IC50 value of 200 nM in cells. Through medicinal chemistry optimization, we developed SI-2, which selectively reduces SRC-3 protein levels and is highly potent (IC50 3-20 nM) towards killing breast cancer cells while not affecting normal cell viability. In addition, SI-2 significantly inhibits primary tumor growth and reduces SRC-3 protein levels in a breast cancer mouse xenograft model. In toxicology studies, we found that SI-2 causes no observable acute or chronic toxicity to major organs in vivo (PNAS 2016). One of the caveats is that SI-2 has a relatively short plasma half-life (1 h). We identified the metabolic “soft spot” of SI-2 and developed SI-X that has similar biological activities to SI-2 but significantly longer plasma half-life (6 h). Additionally, SI-X has ~40% oral bioavailability. Our pilot study demonstrated that SI-X can significantly inhibit tumor growth in an orthotopic pancreatic cancer mouse model. We envision that based on the SI-2 class of compounds we can develop the first clinically testable SRC-3 SMI and expand the arsenal to fight cancer.
Enhancing Intracellular Accumulation and Target Engagement of Proteolysis Targeting Chimeras (PROTACs)
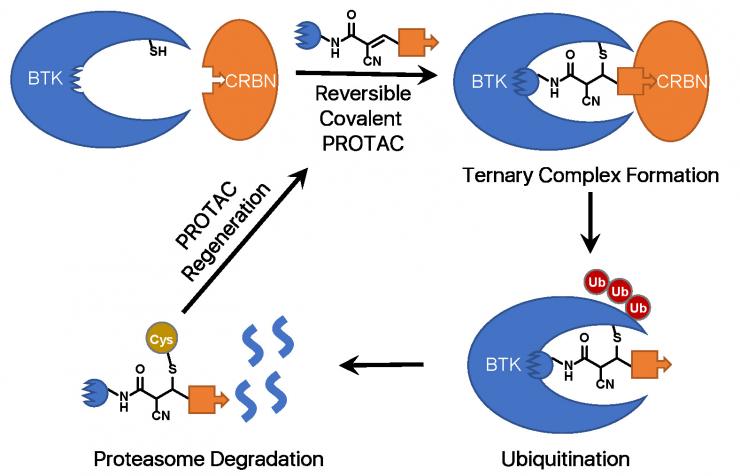
A PROTAC is a heterobifunctional molecule that can bind both a target protein and an E3 ubiquitin ligase to facilitate the formation of a ternary complex, leading to ubiquitination and ultimate degradation of the target protein. Current efforts in the PROTAC field mostly focus on choosing an appropriate E3 ligase for the target protein, improving the binding affinities towards the target protein and the E3 ligase, and optimizing the PROTAC linker. However, due to the large molecular weights of PROTACs (generally > 800 Da), their cellular uptake remains an issue.
Through comparing how different warhead chemistry, reversible noncovalent (RNC), reversible covalent (RC), and irreversible covalent (IRC) binders, affects the degradation of Bruton’s Tyrosine Kinase (BTK), we serendipitously discover that cyano-acrylamide-based reversible covalent chemistry can significantly enhance the intracellular accumulation and target engagement of PROTACs and develop RC-1 as a reversible covalent BTK PROTAC with a high target occupancy as its corresponding kinase inhibitor and effectiveness as a dual functional inhibitor and degrader, providing a novel mechanism-of-action for PROTACs (Nature Communications 2020).
Importantly, this reversible covalent strategy can be generalized and applied to improve other PROTACs. This work can not only help to develop optimal BTK degraders for clinical applications but also provide a new strategy to improve PROTAC efficacy. This work led to a patent application and a recently funded R01 grant score at 1%.
Novel Soluble Epoxide Hydrolase Inhibitors for the Treatment of Alzheimer's Disease
Alzheimer’s disease (AD) is the most common cause of dementia and one of the leading causes of death in the United States. AD is the only leading cause of death for which no disease-modifying therapy is currently available. Neuroinflammation plays a major role in AD pathogenesis. Epoxyeicosanoid signaling is a key integrator of cell-cell communication in the central nervous system (CNS), coordinating cellular responses across different cell types. Epoxyeicosatrienoic acids (EETs) are arachidonic acid metabolites of cytochrome P450 epoxygenase that have potent anti-inflammatory activity.
In collaboration with Dr. Zheng’s group at Baylor, we demonstrated that pharmacological inhibition of sEH can attenuate neuroinflammation, enhance reduction of plaque pathology, and eventually reverse spatial learning and memory deficits in preclinical models of AD. Although some sEH inhibitors (sEHIs) have been reported, none of them are optimized for CNS applications. Blood brain barrier (BBB) is the main hurdle for CNS drug development. Taking advantage of high throughput virtual screening, we identified a novel and patentable pharmacophore for sEHIs, which is different from previous urea- or amide-based sEHIs.
Through further medicinal chemistry optimization, we developed a lead compound with potent inhibitory activity against sEH in both enzyme and cell-based assays. The lead compound attenuates the inflammatory response in LPS-stimulated primary astrocytes, shows ideal in vivo PK, oral bioavailability and CNS penetration, and reduces LPS-induced neuroinflammation in vivo. A provisional patent covering this novel class of sEH inhibitors has been filed. We are also awarded a U01 grant from NIH/NIA to support a comprehensive medicinal chemistry campaign and all the pre-Investigation New Drug (IND) studies. Successful accomplishment of this project will open a new avenue for treating and preventing AD and advance our scientific knowledge of multiple mechanisms of AD.








 Credit
Credit