Functional Mapping of Central Noradrenergic Neurons in the Control of Breathing
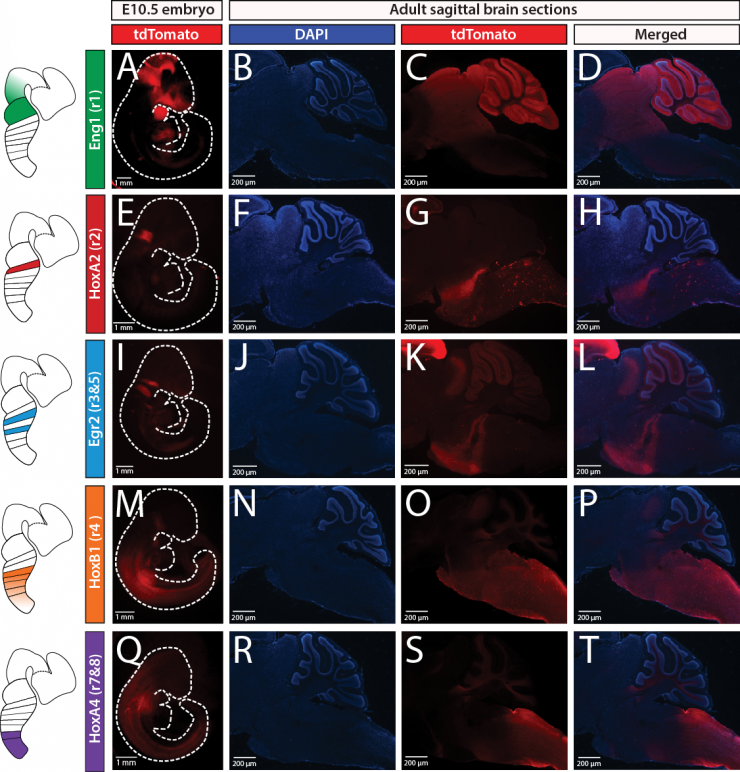
Several respiratory pathophysiologies including SIDS, Rett syndrome, and CCHS feature both disordered breathing and are associated with brainstem noradrenergic system (NA) abnormalities. However, the central NA system is diverse, spread across 10 anatomically distinct nuclei in the brainstem, and sends projections throughout the nervous system to server a multitude of behavioral and physiological functions. Thus, it remains unclear how the central NA system is organized to regulate breathing and how this NA neurons may play a role in pathophysiologies such as SIDS and Rett Syndrome. To determine the role of the central NA system in respiratory control and disease, we have built a number of dual recombinase intersectional genetic tools for precise and non-invasive circuit mapping and pair these models with our custom designed equipment to measure cardio-respiratory and metabolic function in mice.
Screening for Genes and Prenatal Exposures in Sudden Infant Death Syndrome
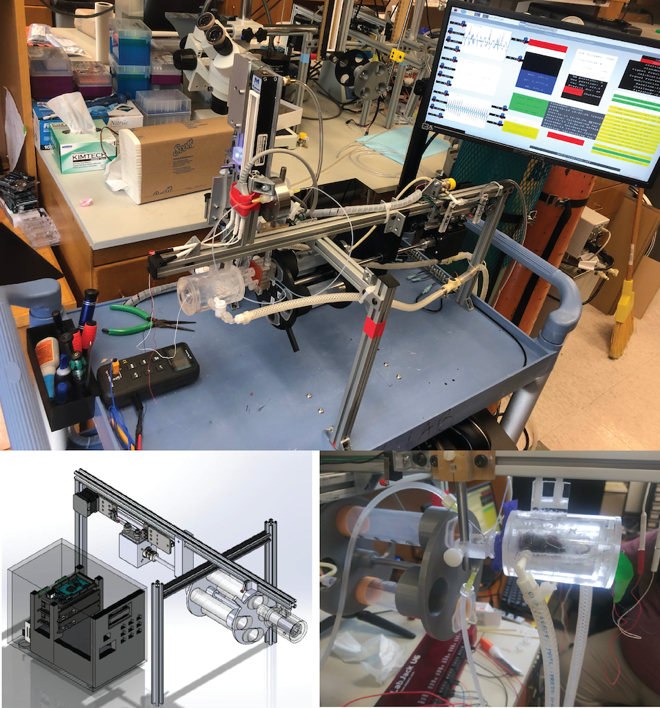
Sudden Infant Death Syndrome claims the lives of as many as 14 infants each day in the United States alone. SIDS is typically defined as the death of an otherwise healthy infant, which leave few clues to understand how this devastating disease may occur. However, carefully controlled post-mortem studies have identified subtle brainstem abnormalities in areas critical to breathing. It remains unclear if these subtle abnormalities stem from genetic mutations, pre-natal exposures such as smoking and alcohol or both. To answer that question, we have developed a closed loop automated neonate respiratory measurement platform. View video. Using these robotic platforms, we are able to simultaneously test the breathing of large numbers of neonate mice under SIDS like conditions. We are currently leveraging these platforms to first screen a wide array mouse genetic mutants for SIDS like phenotypes and as well as to better understand how pre-natal exposures including alcohol, tobacco, stress, and diet and influence protective neonate respiratory reflexes.
COVID-19 and the Effects of SARS-CoV-2 Infection on the Nervous System
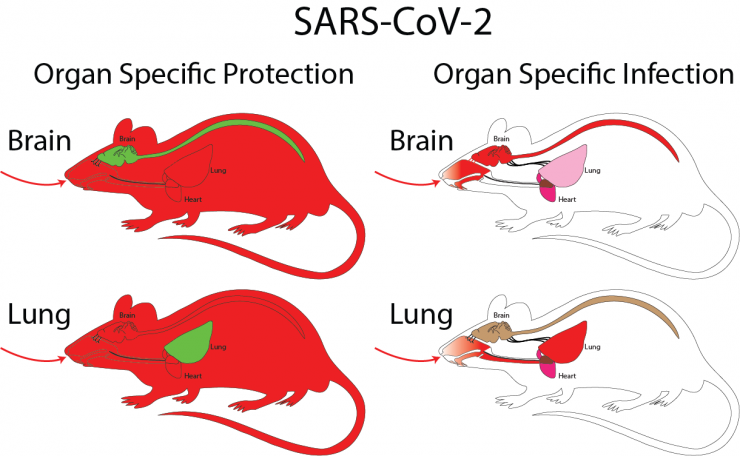
The current pandemic has revealed a multitude neurological sequelae in both the acute phase of infection and long lasting after the infection has cleared (Long COVID). It remains unclear whether these effects are the result of direct nervous system infection or stem from indirect inflammatory effects, neuro-vascular damage, or PAMPS/DAMPS (Pathogen associated molecular patterns or Damage associated molecular patterns) crossing the blood brain barrier. To address these questions and to determine the direct and indirect effects of SARS-CoV-2 infection on nervous system function, we have engineered a set of genetically modified mouse mutants that allow for the isolated infection a specifically targeted cell type or organ protecting the rest of the body by conditional hACE2 expression. Conversely, we also genetically engineered mouse models that protect targeted cell types or organs while the rest of the animal can be infected. To assess the role of brain specific infection or protection in SARS-CoV-2 infection, we have also engineered a novel BSL3 cardio-respiratory plethysmograph and metabolic system (for VCO2 and VO2) enabling precise physiological assessments in disease progression in COVID-19 studies.








