Research Questions
Recognition of Dying Cells
- What is the molecular nature of the "eat me" signal(s) presented on the surface of apoptotic and necrotic cells?
- What is the molecular mechanism that leads to the presentation of an “eat me” signal to the surface of apoptotic cells?
- How do cytoplasmic Ca2+induces necrotic cells to expose phosphatidylserine (PS), the “eat me” signal that attracts engulfing cells?
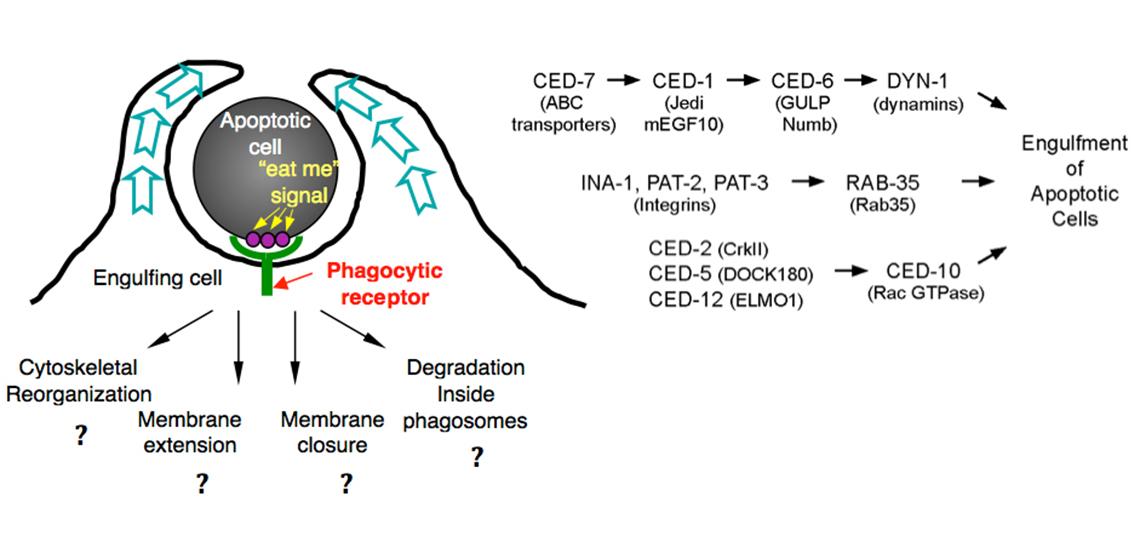
- How does the phagocytic receptor CED-1 recognize dying cells? Besides CED-1, is there another phagocytic receptor that recognizes dying cells?
Engulfment of Dying Dells
- How is CED-1 activated by its association with apoptotic cells?
- What are the signaling pathways that trigger and regulate the polarized extension of engulfing cell surfaces that embrace the apoptotic cells?
- How are the plasma membrane and the actin cytoskeleton underneath it rearranged during cell-surface extension?
- How is membrane trafficking involved in the engulfment of apoptotic cells?
Degradation of Dying Cells
- What are the molecular events, in particular membrane trafficking events, that are involved in the degradation of apoptotic cells? Organelles in the endocytic pathway, such as early endosomes and lysosomes, are known to fuse with phagosomes and deliver digestive enzymes to phagosomal lumen. Are there other kinds of intracellular organelles that fuse to phagosomes and promote phagosomal degradation?
- How does the autophagy pathway interact with the phagocytosis pathway?
- What are the signaling molecules that trigger the recruitment and fusion of intracellular organelles to phagosomes? What are the effectors of these signaling molecules? How do the effectors work on the molecular level?
- How are the nutrients from degraded dying cells recycled inside host cells?
Clearance of Apoptotic Cells and Environmental Stresses
- How do engulfing cells cope with environmental stresses during the clearance of dying cells?
- What is the molecular mechanism behind the function of RAB-35, which is a robustness factor that functions to stabilize the apoptotic cell-clearance machinery under high temperature?
- What are the identities of the members of the RAB-35 pathway?
Research to Answer These Questions
To answer these questions, we have established effective experimental systems that include forward genetics screens for mutants defective in each one of the three steps of dying cell removal, genetic and molecular analyses of gene functions, and many cell biological tools, in particular the time-lapse fluorescence microscopy technique, which allows us to visualize many subcellular events that occur during the process of dying cell removal in developing C. elegans embryos in real time. The combination of genetic, cell biological, and molecular biological tools has enabled us to discover an “eat me” signal presented on the surface of both apoptotic and necrotic cells for attracting engulfing cells, and multiple proteins that are essential for the engulfment of dying cells. Furthermore, we successfully used these methods to identify multiple signaling molecules and their effectors that drive phagosome maturation through a signaling pathway initiated by the activation of CED-1 on nascent phagosomes and mediated by the large GTPase dynamin.
In summary, the recognition, engulfment, and degradation of apoptotic cells are three integral steps in the process of apoptotic-cell removal. C. elegans is a particular good system for studying the molecular mechanisms behind each step due to the existence of powerful tools for genetic and cell biological analyses. Continuing our research using C. elegans as a model organism will allow us to discover the molecular mechanisms that control each cellular event related to the removal of dying cells, and will further provide clues for us to expand our research into the phagocytic removal of other targets, such as invading pathogens.