Our Research
The Laboratory of Epilepsy and Emotional Behavior, led by Vaishnav Krishnan, M.D., Ph.D., is devoted to advancing our knowledge of the basic neurobiological mechanisms that underlie the strong associations between epilepsy and mental health disorders.
The central objective of the laboratory is to dissect the basic mechanisms by which specific epilepsies (in which hyperexcitability may be genetic, lesional or other) result in specific derangements in behavior. Using a range of genetically valid mouse models of epilepsy (or epilepsy risk), we employ state of the art home cage behavioral monitoring technology to understand how interictal behavior is directly modulated by latent genetic risk, induced seizures, frank spontaneous seizures and/or anticonvulsants. In contrast to standard behavioral phenotyping batteries, all of our behavioral measurements are conducted within the mouse’s home cage.
This provides a particularly unique window into understanding exactly what mice do at night during their “active” phase. By applying high resolution video tracking within instrumented home cage chambers, we simultaneously assay at least SIX quantities (horizontal movement, “sleep”, sheltering, drinking, eating and wheel running) over prolonged recording periods (23-24h). Thus, we make no assumptions about when or how a manipulation (genetic or pharmacological) may be “symptomatic”. We closely examine undisturbed “baseline” behavior, as well as behavioral responses to certain provocative maneuvers, like a light spot, and we study both mice that are housed individually or in pairs (“dyads”).
By simultaneously incorporating wireless EEG, we are able to gain insights into the behavioral correlates of ictal and post-ictal behavior, as well as signatures of “pre-ictal” behavior, which we will apply to devise algorithms for seizure prediction or seizure forecasting. Our work in mice directly translates to a large body of ongoing research designed to objectively and quantitatively describe neuropsychiatric disability through the use of wearable devices (wrist/waist accelerometers/smart phone apps) that continuously assess “mood” and “activity” in situ.

Behavioral Monitoring Unit
Our BMU, or Behavioral Monitoring Unit, comprised of 16 instrumented home cage chambers. Temperature and humidity settings, food/water/bedding, as well as the light cycle (12:12 OFF at 1700) are all matched to vivarium conditions. Mice are permitted to shelter within an infrared lucent compartment. Two separate lickometered water spouts measure water and sucrose-water consumption. Aerial infrared cameras permit continuous video tracking of single or pairs of mice regardless of ambient light conditions. A food meter (designed with a beam break device) measures feeding, and wheel running is measured via a detachable running wheel.
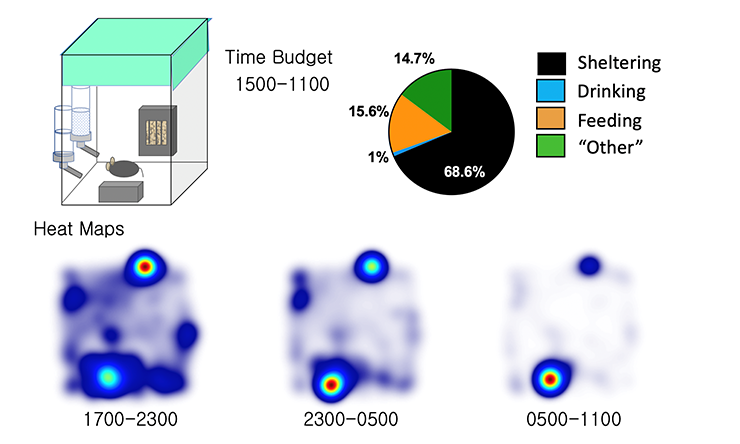
Data
In addition to time series data, our platform also enables the measurements of time budgets, providing a top down view of spontaneous behavioral choices over prolonged recording periods. Heat maps depicting peaks of position probability provide a pictorial representation of spontaneous home cage behavior.

Clusters of Behavioral or Psychiatric Comorbidity in Epilepsy
While seizures are the defining characteristic of the “epilepsies”, seizures often present in the context of neuropsychiatric symptoms that can be organized into symptom clusters. These symptom clusters reflect the unique ways in specific causes of hyperexcitability result in specific derangements in interictal behavior. With access to a range of genetic mouse models of epilepsy or epilepsy risk, we have discovered unique interictal behavioral derangements in each of these models. Not surprisingly, our most interesting phenotypes emerge at night.
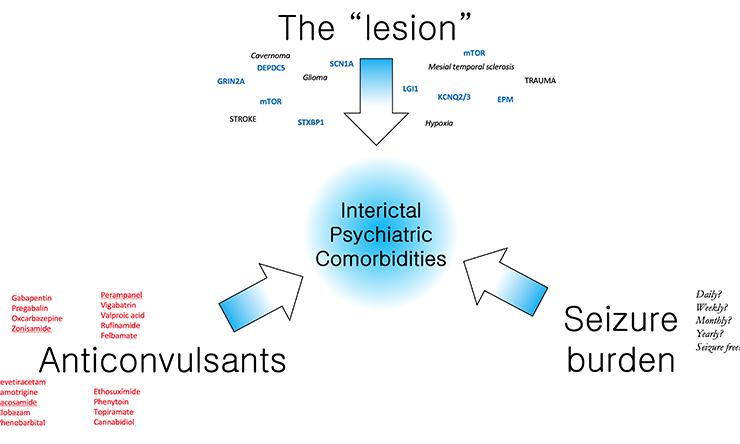
Modulators of Interictal Behavior
A working model to dissect the neurobiology of interictal behavior. We propose that in patients with epilepsy, “net” derangements in cognitive and emotional behavior reflect the summated contributions of three main elements, including the “lesion”, the burden of long term anticonvulsant use and the burden of prior seizures. By selectively manipulating these elements individually in genetically valid mouse models, the Krishnan lab aims to delineate a systematic approach to assessing the etiology of psychiatric comorbidity.
Epilepsy Spectrum Disorders
Epilepsy, or the epilepsies, are an etiologically heterogeneous collection of diseases defined by (i) an enduring predisposition to epileptic seizures and (ii) by associated cognitive, psychological and social consequences. Increasingly, epilepsy has been recognized as a spectrum disorder that reaches well beyond seizures: nearly half of all patients with epilepsy experience “interictal” symptoms that range from mild impairments in attention and memory, to pervasive emotional derangements such as depression, autism, psychosis or anxiety disorders that may be complicated by suicidality. The occurrence of these psychiatric comorbidities in patients with epilepsy results in greater impairments in overall quality of life, increased health care utilization and costs, and also complicate the selection of medications or surgical treatments designed to prevent seizures. While the severity of these cognitive and emotional derangements are generally proportional to seizure frequency, epidemiological data suggest that shared pathophysiological mechanisms may coordinately enhance seizure risk (ictal disability) and pervasively disrupt limbic function (interictal disability). Thus, it’s not that seizures necessarily cause depression, autism or anxiety. Instead, comorbid depression, autism or anxiety symptoms are likely a result of a broad brain network derangement which is secondary to the epileptogenic lesion itself (which may be a genetic mutation, an old stroke or scar, a brain tumor or entirely unknown). Indeed, psychiatric comorbidities such as depression or autism may arise up to years prior to the occurrence of the first seizure.
Research Support
Our work has been generously supported by the Baylor College of Medicine Office of Research, the American Academy of Neurology, the American Epilepsy Society and the NINDS. We are also fortunate to be engaged in strong bidirectional scientific collaborations with a range of epilepsy-focused laboratories on and off-campus, including Dr. Jeffrey Noebels, Dr. Atul Maheshwari, Dr. Edward Cooper, Dr. Jeannie Chin and Dr. Berge Minassian.








