
The scientific focus of Dr. Marchetti’s laboratory is penetrating research to improve understandings of mechanisms underlying brain metastasis; this, by capturing changes in the brain microenvironment and investigating determinants implicated in the early events of tumor cell colonization to brain.
The ultimate objective is to apply concepts and findings clinically for novel and effective treatments combating brain metastasis.
Research Objectives
The following are research aims for the Marchetti Lab:
Heparanase
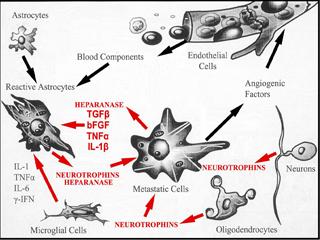
Determine how heparanase, a master regulator of cancer metastasis, affects the onset of brain metastasis via enzymatic and non - enzymatic functions, and at multiple levels, e.g., tumor cell adhesion, vascular cooption, disruption of the blood-brain-barrier, cytoskeletal dynamics, tumor cell extravasation/growth, and promoting chemotherapy resistance.
Brain metastasis remains the most devastating and feared consequence of cancer with more than 40 percent of all cancer patients developing brain metastasis. For example, patients with brain metastatic breast cancer (BMBC) have, even with the best available treatments, only a 20 percent one-year survival rate. BMBC is particularly common in breast cancers that are positive for epidermal growth factor receptor1 and 2 (EGFR and HER2), however, targeted therapies against BMBC, e.g., lapatinib - a EGFR/HER2 dual kinase inhibitor, are only minimally effective with response rates lower than those for extracranial metastases. The identification of mechanisms responsible for BMBC is imperative to develop new therapies.
By several pre-clinical studies, my laboratories have demonstrated that heparanase (HPSE) acts as a potent pro-tumorigenic, pro-angiogenic, and pro-metastatic enzyme. Heparanase is the only endoglycosidase in mammals which cleaves heparan sulfate (HS), the main polysaccharide of cell surface and tumor-surrounding extracellular matrix, into fragments retaining biological activity. An established role for heparanase is to release HS-bound growth and angiogenic factors stored in the extracellular matrix, and regulate their levels and potency, thus initiating many effects which drastically alter the metastatic outcome. These functions are mediated by enzymatically active heparanase. However, recent findings indicate that heparanase possesses functions which are independent of its enzymatic activity, and acts as an adhesion molecule and signal transducer. The therapeutic disruption of heparanase therefore provides an opportunity to block multiple pathways that control tumor-host interactions and are crucial for tumor growth and metastasis.
Our work has implicated heparanase as a promoter of brain metastasis whose activity is highest in cells metastasizing to brain. The enzyme is also produced by cells of the brain microenvironment in response to a brain neoplastic insult fostering metastatic growth. Further, we have demonstrated that heparanase is expressed in BMBC tissues, and functions as a target of EGFR/HER2 pathways affecting BMBC cell proliferation. Overall, these findings set the stage for developing therapies aimed at the heparanase/HS axis. To move toward this goal, we must gain a better understanding of the mechanisms of heparanase, which are poorly understood. By studying much broader roles for heparanase, which involve enzymatic and non-enzymatic functions, and deciphering these roles at distinct steps of brain colonization, we aim to demonstrate that heparanase regulates the cross-talk between BMBC and cells of the brain microenvironment, and initiates multiple effects which are critical for the development and progression of BMBC.
Circulating Tumor Cells
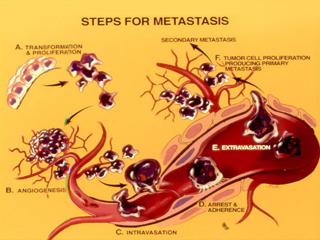
Identify subsets, and investigate properties and molecular signatures of Circulating Tumor Cells (CTCs) - the "seeds" of uncurable metastatic disease - as unique alternative to invasive biopsies for the detection, diagnosis, and monitoring of tumors with a predilection to metastasize to brain.
The identification and characterization of circulating tumor cells (CTCs) inductive of fatal metastasis remains elusive. Because care is essentially palliative once metastasis occurs and drug combinations are rarely tested to eliminate established metastasis, new approaches to predict metastatic onset for the development of effective treatments are critical ("secondary metastasis" prevention or "metastasis of metastasis"). They can be more profound when coupled with a definition of CTC - associated characteristics.
Specifically, the incidence of brain metastatic breast cancer (BMBC) is alarmingly increasing. BMBC is common in patients negative for estrogen/progesterone receptors and over-expressing epidermal growth factor receptor1 or 2 (EGFR or HER2/neu). However, both traditional and recent therapies using EGFR/HER2 target designs had underwhelming success in the clinical management of BMBC.








