OnCore Clinical Trial Management System

The Dan L Duncan Comprehensive Cancer Center’s Biomedical Informatics Group in the Biostatistics and Informatics Shared Resource maintains OnCore for management of the full lifecycle of clinical trials. This system, considered by many to be the industry gold standard, allows centralization of all your clinical trials data, electronic documents, visit tracking and billing compliance within one database.
For trials that use other systems for data management, such as pharmaceutical sponsor or cooperative group clinical trials management databases, only limited participant accrual data are required to satisfy NCI and other reporting requirements. Many investigator-initiated protocols or those where BCM is the data coordinating center need “full build” with electronic case report forms, electronic calendars, quality assurance, regulatory management, and billing compliance. The BISR and Clinical Trials Support Unit support both of these scenarios.
If you would like to utilize OnCore for an upcoming clinical trial, please contact oncore-support@bcm.edu.
Service and Support
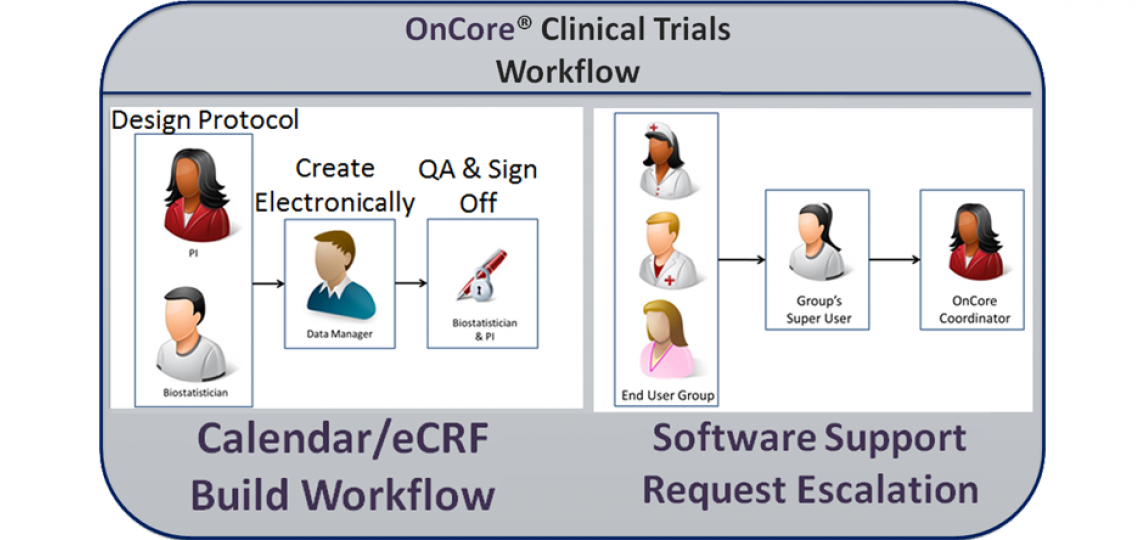
Trial Creation
The OnCore team strongly encourages PIs to work with their Cancer Center program staff biostatistician (see the table below) as early as possible during initial study design phases to discuss the statistical plan and define data collection forms. The biostatistician will work with you, the PI, to create a final paper version of the protocol documents including forms and a protocol calendar. As the protocol is finalized, your regulatory coordinator should log in to OnCore to enter basic data about the study.
Once the trial is created in OnCore and forms/calendar are finalized on paper, please email the protocol, forms and any ancillary information to oncore-support@bcm.edu, copying your biostatistician. The OnCore data manager will review the paper forms, ask any questions, and then create custom electronic case report forms (eCRFs) and an electronic protocol calendar for the study.
When the data manager is finished, the biostatistician will validate and your study staff will test the eCRFs and calendar. When finalized the PI will give final approval.
| Biostatistician | Programs | |
|---|---|---|
| Susan Hilsenbeck | Center for Cell and Gene Therapy Clinical Trials Support Unit Elkins Pancreas Center Lester and Sue Smith Breast Center Scott Department of Urology Texas Children's Cancer Center | |
| Anna Frolov | Clinical Trials Support Unit Scott Department of Urology | |
| Eunji Jo | Center for Cell and Gene Therapy Elkins Pancreas Center Lester and Sue Smith Breast Center Texas Children's Cancer Center | |
| Tao Wang | Lester and Sue Smith Breast Center | |
| Jessie Wu | Center for Cell and Gene Therapy |
Troubleshooting
The OnCore Coordinator regularly trains end users for basic protocol creation (regulatory staff), basic participant data entry (clinical research coordinators, CRCs), full electronic case report form and calendar visit data entry (CRCs and protocol managers), PI-level case report form use, and beyond.
As end users need assistance, they reach out first to their assigned group's Super User who has received specialized training in OnCore. If the Super User cannot address the end user’s concern or the group does not have a Super User, they can escalate the issue to the OnCore Coordinator and OnCore Support by contacting OnCore-Support@bcm.edu.
Biomedical Informatics Group
Jewish Building, 5th Floor Baylor College of Medicine
One Baylor Plaza
Houston, TX 77030








