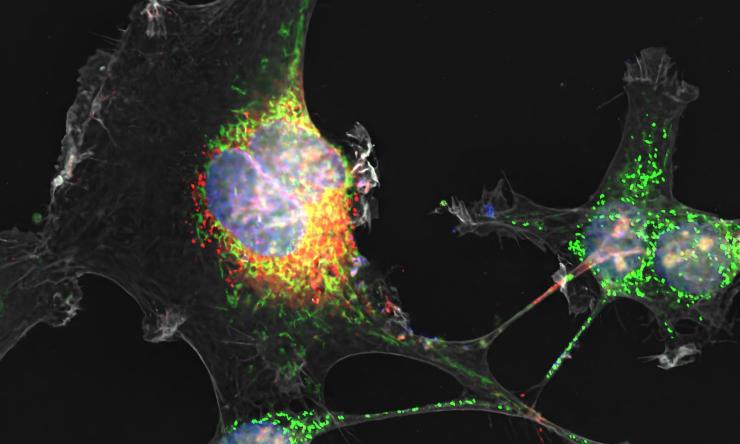
Stealth virus: Zika virus builds tunnels to covertly infect cells of the placenta
Infection with Zika virus in pregnancy can lead to neurological disorders, fetal abnormalities and fetal death. Until now, how the virus manages to cross the placenta, which nurtures the developing fetus and forms a strong barrier against microbes and chemicals that could harm the fetus, has not been clear. Researchers at Baylor College of Medicine with collaborators at Pennsylvania State University report in Nature Communications a strategy Zika virus uses to covertly spread in placental cells, raising little alarm in the immune system.
“The Zika virus, which is transmitted by mosquitoes, triggered an epidemic in the Americas that began in 2015 and by 2018 had reached as many as 30 million cases,” said co-senior author Dr. Indira Mysorekar, E.I. Wagner Endowed, M.D., Chair Internal Medicine II, chief of basic and translational research and professor of medicine – infectious diseases at Baylor. “Understanding how Zika virus spreads through the human placenta and reaches the fetus is critical to prevent or control this devastating condition.”
The researchers discovered that Zika virus builds underground tunnels, a series of tiny tubes called tunneling nanotubes, that facilitate the transfer of viral particles to neighboring uninfected cells.
“We discovered that the formation of these tiny tunnels is driven exclusively by a Zika protein called NS1,” said first author Dr. Rafael T. Michita, postdoctoral research associate in the Mysorekar lab. “Exposure of placental cells to the NS1 protein of Zika virus triggers tunnel formation. As the tunnels develop and connect neighboring cells, a path opens for the virus to invade new cells.”
“Zika is the only virus in its family, which includes dengue and West Nile viruses among others, whose NS1 protein triggers the formation of tunnels in multiple cell types,” Michita said. “Other viruses unrelated to Zika, such as HIV, herpes, influenza A and SARS-CoV-2, the virus that causes COVID-19, also can induce tiny tunnels in cells they infect and use the tunnels to spread to uninfected cells. This is the first time that tunneling has been shown by Zika virus infection in placental cells.”
Interestingly, the tiny conduits provided a means to transport not only viral particles, but also RNA, proteins and mitochondria, a cell’s main source of energy, from infected to neighboring cells. “We propose that transporting mitochondria through the tunnels may provide an energetic boost to virus-infected cells to promote viral replication,” said co-author Long B. Tran, a graduate student in the Mysorekar lab.
“We also show that travelling through the tiny tunnels can potentially help Zika virus avoid the activation of large-scale antiviral responses, such as interferon lambda (IFN-lambda) defenses implemented by the placenta,” Michita said. “Mutant Zika viruses that do not make tiny tunnels induce robust antiviral IFN-lambda response that can potentially limit the spread of the virus.”
“Altogether, we show that Zika virus uses a tunneling strategy to covertly spread the infection in the placenta while hijacking mitochondria to augment its propagation and survival. We propose that this strategy also protects the virus from the immune response,” Mysorekar said. “These findings offer vital insights that could be used to develop therapeutic strategies targeted against this stealth transmission mode.”
Steven J. Bark and Deepak Kumar at Baylor College of Medicine and Shay A. Toner, Joyce Jose and co-senior author Anoop Narayanan at Pennsylvania State University are key members of the research team.
This work was supported in part by grants from NIH/NIAID (R01AI176505), NIH/NICHD (R01HD091218) and Pennsylvania State University startup funds. This project was also supported by the Cytometry and Cell Sorting Core at Baylor College of Medicine with funding from the CPRIT Core Facility Support Award CPRIT-RP180672 and the NIH (CA125123 and RR024574).







 Credit
Credit