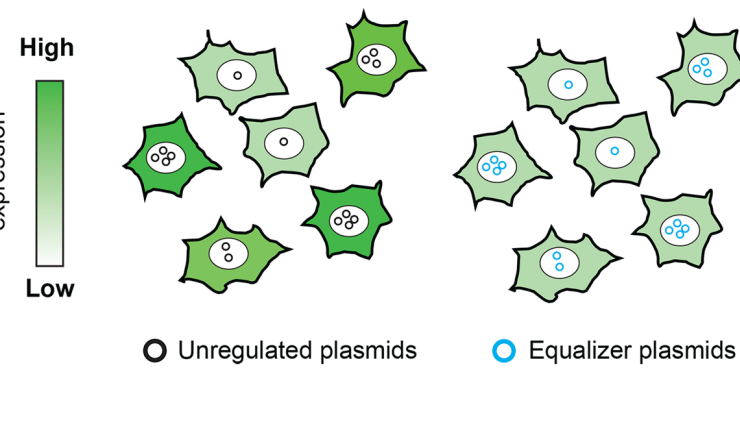
The Equalizer: An engineered circuit for uniform gene expression
The function of a protein can depend on its abundance in a cell. So, when investigating the properties of a new protein, it is essential to make sure that the same amount is produced by every cell. Researchers at Baylor College of Medicine and Rice University have found a new way to do just that through the creation of new genetic circuits called Equalizers.
The findings, in the current edition of Nature Communications, show how researchers engineered these genetic circuits to buffer protein output from variations in the number of copies of the gene inside the cell, thereby helping to create consistent protein expression. This property is called “gene dosage compensation.”
The researchers use an analogy of heating a house to help explain how Equalizers work. Imagine using randomly placed space heaters to heat your home. To ensure each room gets a heater you would purchase some extra ones, but that would mean some rooms might have extra heaters. Those rooms might be too hot, so a solution would be to have thermostats on each heater to downregulate the heat when a room becomes too hot. Those thermostats act as the Equalizer.
Researchers typically encode genes to be expressed on circular pieces of DNA called plasmids. Excess plasmids are often used to ensure that most cells get one, but some cells will get several. The Equalizer is composed of transcriptional negative feedback and post-transcriptional incoherent feedforward loops. These loops counteract the presence of extra plasmids: they sense the outputs, in this case the proteins and mRNAs that the plasmids produce, and tune down their expression if they rise too high.
“We didn’t invent the parts, but rather we invented a new way to connect them together into a circuit,” said Jin Yang, who shared co-first authorship of the paper with graduate student Jihwan Lee of Rice University. Jin was a bioengineering undergrad at Rice University while developing this work and currently is a Ph.D. student at the Massachusetts Institute of Technology. “In natural systems, some gene networks must control gene dosage variation to remain functional and conserve their properties.
We repurposed and combined two types of gene dosage compensation circuits to create a version that enables uniform expression of any protein scientists want to produce in the lab.”
Negative feedback and incoherent feedforward circuit subcircuits can each help compensate for gene dosage, but the researchers found that coupling the two improved overall performance. This is because each circuit is not perfect. For example, the incoherent feedforward loop can saturate because it requires other proteins that are present in limited quantities in the cell. The negative feedback loop is limited in its inhibitory capacity, similar to a leaky sink faucet that cannot be fully closed. But combining these two imperfect circuits produced robust performance, with each circuit helping mitigate the limitation of the other.
“The process we used for these findings was a collaborative effort bringing together computer simulations and biology. This is similar to how engineers work – they draw up their plans, create a model and then build their structure,” said Dr. Oleg Igoshin, professor of bioengineering, of biosciences and of chemistry at Rice University and a senior author on the paper. “In this case we were able to create our model and show the effectiveness through computational models before it was then synthetically engineered in the lab.”
But why is minimizing expression variation important when it comes to biological research?
“The effect of a protein can depend on its abundance in a cell. If you’re studying a new protein and its concentration is too low, you may not be able to observe its function in the cell. If its concentration is too high, the protein may mislocalize, aggregate, produce cytotoxicity or otherwise produce responses that are not physiological. It is therefore important that a protein is expressed at the desired level in every cell under investigation,” said Dr. François St-Pierre, assistant professor of neuroscience and McNair Scholar at Baylor and corresponding author of this study. “We believe Equalizers will be of high value both for basic research and for industry.”
Others who took part in this research include Michelle A. Land and Shujuan Lai, both with Baylor College of Medicine.
The research was supported by the Welch Foundation, a Klingenstein-Simons Fellowship Award in Neuroscience, the McNair Medical Foundation, the National Science Foundation (including the NSF-supported Center for Theoretical Biological Physics), the National Institutes of Health, and the Cancer Prevention and Research Institute of Texas.
Read the full study here.
DOI: 10.1038/s41467-021-23889-0