Regulation of Potassium Channel Assembly and Function by the N-terminal T1 domain
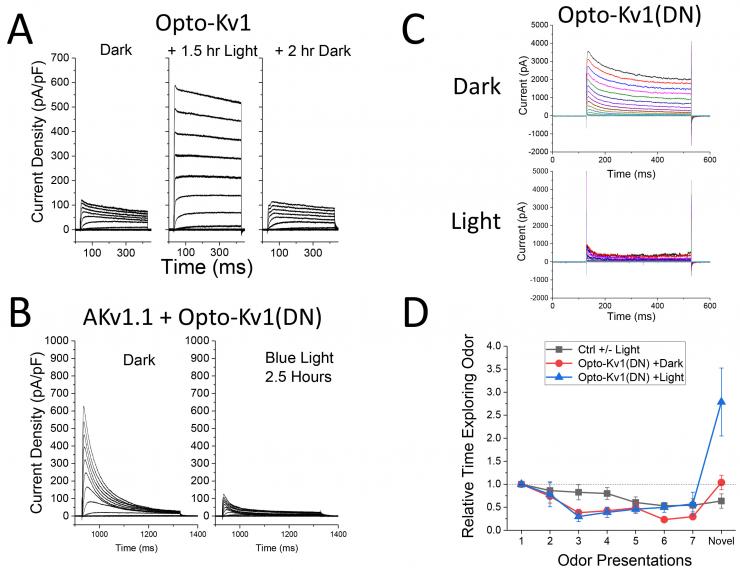
Our research showed that the N-terminus of voltage-gated potassium channel subunits controls the assembly of these proteins into a functional channel. Recently, we have used this fact to regulate potassium channel expression in neurons in a light dependent manner. Structural modeling was used to insert a LOV domain next to the T1 domain to create Opto-Kv channels. LOV domains change their structure following absorption of blue wavelength photons. Using the design we developed, we could demonstrate light dependent regulation of voltage-gated potassium channel formation. In addition, we showed that insertion of a dominant negative pore mutation into these constructs results in a subunit (Opto-Kv(DN)) that knocks down wild type channel expression in a light dependent manner. Recently, we have found that these constructs can be used to regulate animal behavior.
Regulation of Potassium Channels by Auxiliary Subunit Proteins
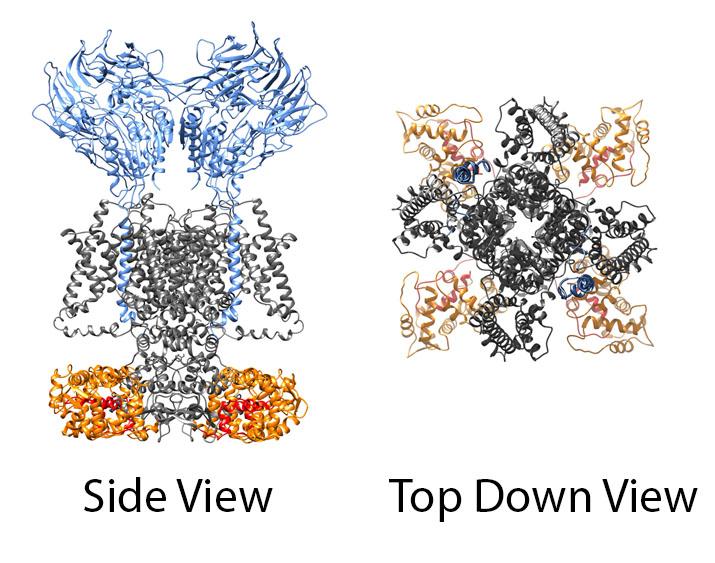
Potassium channels are complex molecular machines whose function, targeting and regulation is strongly influenced by the presence of auxiliary subunit proteins. We have been characterizing many of the interacting proteins to determine how they interact with the channels as well as the functional, targeting, and regulatory effects they produce.
Understanding Potassium Channel Inactivation and its Role in Nervous System Function
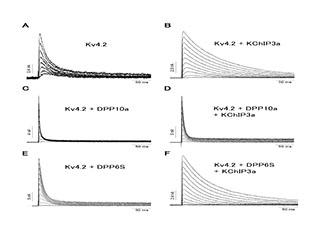
Inactivation is the process by which a channel shuts down in the face of repeated stimulation to be active. This kinetics and extent of this process varies dramatically between different channels and is under the regulation of kinases, reactive oxygen species and auxiliary subunit proteins. Unlike sodium channel inactivation, which acts to silence neurons, potassium channel inactivation produces an opposite effect, essentially producing a window of increased excitability. We believe that this window likely serves as an associative mechanism by allowing one signal to alter the effects of a second signal that arrives during the window of inactivation. We have been performing studies to characterize the mechanisms underlying inactivation as well as how varying the inactivation properties of channels in neurons affects their function.
Improving Methods to Computationally Analyze Roles of Specific Ion Channels in Nervous System Function
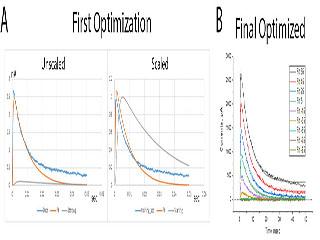
We have been working on developing approaches to ion channel modeling using modern Neural Network programming toolboxes like PyTorch and TensorFlow. Previous generation computational tools could be used to fit data, but were difficult to extract a detailed understanding of how different channel parameters related to neuron and neural network function. Using our new approach, we can globally fit channel gating recorded under a variety of conditions and then use powerful analysis routines incorporated in the software to analyze the exact impact of any parameter on our model’s function.








