Role of the Gut Microbiota and Inflammation in Endometriosis
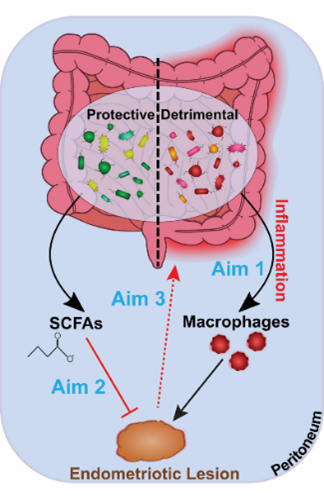
Endometriosis, which causes pain in the pelvis and lower abdomen and can lead to infertility, afflicts 1 in 10 women in reproductive age. A well-accepted theory is that endometriosis occurs when endometrial tissue enters the peritoneal cavity via retrograde menstruation and implants onto pelvic organs. However, whereas up to 90% of women experience retrograde menstruation, only 10% of women develop endometriosis, suggesting that unknown factors contribute to development and progression of endometriosis.
Considered a central player of hormone regulation and inflammation, we are interested in investigating the role of gut microbiota and its derived metabolites in this debilitating condition.
We focus on understanding the molecular factors that are involved in endometriosis and are regulated by the gut microbes to address our questions on their bidirectional relationship.
Role of RNA Splicing in Gynecological Pathologies
At Kommagani lab, we focus on another common pathology of the female reproductive tract, the endometrial (uterine) cancer. The condition has a five-year survival rate of only 17% and at present, few treatment options are available for the ~9% of endometrial cancer patients who have late-stage metastatic disease. Our goal is to identify new prognostic and therapeutic targets to reduce the death toll of this disease. Towards this goal, we reported an increase in the splicing factors during endometrial tumorigenesis that is regulated by steroid hormones. We are interested in dissecting these mRNA splicing events to understand the pathobiology of endometrial cancer beyond the transcriptional effects of steroid hormones.
We now have a four-year grant from the American Cancer Society to identify the mRNA splicing alterations caused by overexpression or increased activity of SF3B1 that promote endometrial cancer progression. Currently, we are generating uterine-specific heterozygous Sf3b1 knockout mice to assess the role of SF3B1 in estrogen-driven endometrial hyperplasia in vivo. Long term, this project may lead to exploring estrogen-induced alternative splicing in other gynecological pathologies, including endometriosis.
Role of RNA Splicing and Autophagy in Endometrial Preparation for Embryo Implantation
Another area of our interest is in the implantation failure and early pregnancy loss in women. We are interested in the fascinating area of research on understanding the events that prepare the uterine endometrium for implantation. Specifically, we are investigating the roles of RNA splicing and autophagy as two crucial pathways in regulating endometrial physiology and homeostasis.
RNA splicing: We recently highlighted important role of splicing factors in endometrial decidualization. Currently we are testing our hypothesis that the splicing factors and their mediated events are essential for endometrial function pivotal to successful implantation. We plan to identify the progesterone-induced RNA splicing events and the splicing factors that are involved in the coordinated sequence of events during embryo implantation.
Autophagy: Endometrial changes are often correlated with autophagy that are required for endometrial homeostasis. We have observed implantation defects and thus, reduced fecundity in conditional knockout mice models of the autophagy-related proteins in the reproductive tract. In the absence of these proteins, we observed aberrations in progesterone signaling and stroma formation and an intact epithelium in the postnatal uteri. We hypothesize that some of these autophagy related proteins are crucial for the maintenance of progenitor stem cells in endometrium and thus, we are interested in evaluating the underlying molecular mechanism of the functions of autophagy related proteins in endometrial tissue development and in delineating their autophagy and non-autophagy driven functions during endometrium generation.








