Project Co-leaders
- Thomas F. Westbrook, Ph.D. (Basic Science Leader)
- C. Kent Osborne, M.D. (Clinical Science Leader)
- Xiang Zhang, Ph.D. (Basic Science Leader)
Project Description
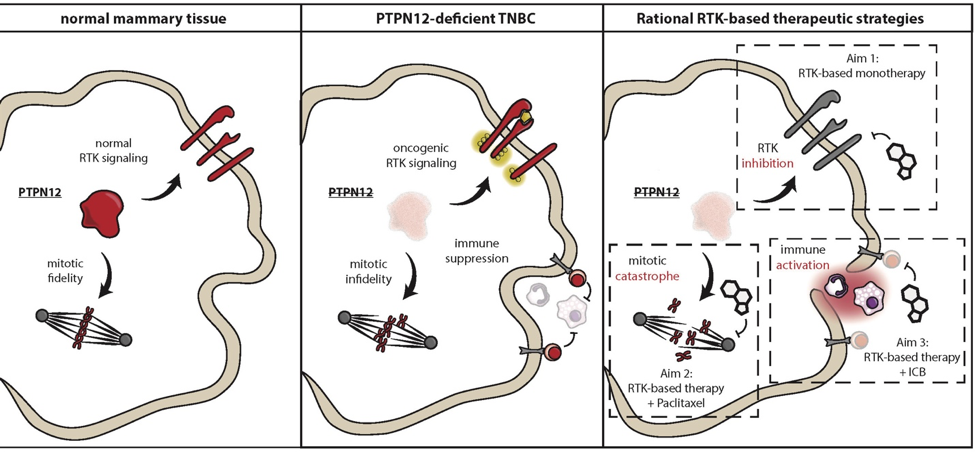
Despite extensive efforts to characterize the genomes and proteomes of triple-negative breast cancer (TNBC), no dominantly-acting mutated RTK has emerged as a therapeutic target in TNBC. Instead, TNBCs exhibit broad, rather than selective, hyper-activation of RTK signaling, with several proto-oncogenic RTKs hyper-activated in the same tumor. Recent discoveries have revealed that the coordinate activation of RTKs observed in TNBC is a result of inactivation of the protein tyrosine phosphatase PTPN12 (ex. Sun et al, Cell; Nair et al, Nature Medicine). In the current proposal, we aim to translate our new knowledge of the mechanisms by which RTKs are aberrantly activated and drive TNBC progression into a new therapeutic approach for TNBC patients.
Data from our team and confirmed by others indicates that PTPN12 functions as a tumor suppressor by restraining the activity of a select set of proto-oncogenic RTKs, including MET and PDGFRB, binding these receptors and suppressing downstream signaling. PTPN12 is compromised in 45-55% of TNBCs, and we have shown in in vitro and pre-clinical models of TNBC that inactivation of PTPN12 leads to hyper-activation of MET, PDGFRB, and a small subset of other RTKs. Importantly, this provokes the therapeutic hypothesis that PTPN12-deficient TNBCs can be treated by combined targeting of this select set of hyper-activated RTKs. To translate these pre-clinical findings into a new therapeutic approach, we will evaluate the efficacy of the well-tolerated MET/PDGFR inhibitor sitravatinib as monotherapy in metastatic TNBC patients, develop rational strategies to combine sitravitinib with standard of care therapies (based on newly discovered synthetic lethalities in TNBC), and explore new ways to capitalize on the anti-tumoral and pro-immune effects of sitravatinib in the context of immune checkpoint therapies.
Herein, our mechanistic, pre-clinical, and clinical studies will evaluate the proof-of-concept for the use of sitravatinib to treat PTPN12-deficient TNBC and lay the groundwork for the next generation of combination approaches for TNBC and other RTK-dependent cancers.