Role of Foxi3 in Craniofacial Development and Ectodermal Placodes
Craniofacial abnormalities are some of the most common birth defects, ranging from cleft lips and palates to more severe disorders such as DiGeorge or Treacher-Collins syndromes. Craniofacial abnormalities often arise during formation of the pharyngeal arches destined to give rise to many craniofacial structures. The complexity of arch derivatives is reflected in their development, which requires an intricate orchestration of interactions between arch ectoderm, endoderm and mesoderm, together with neural crest cells that populate each arch.
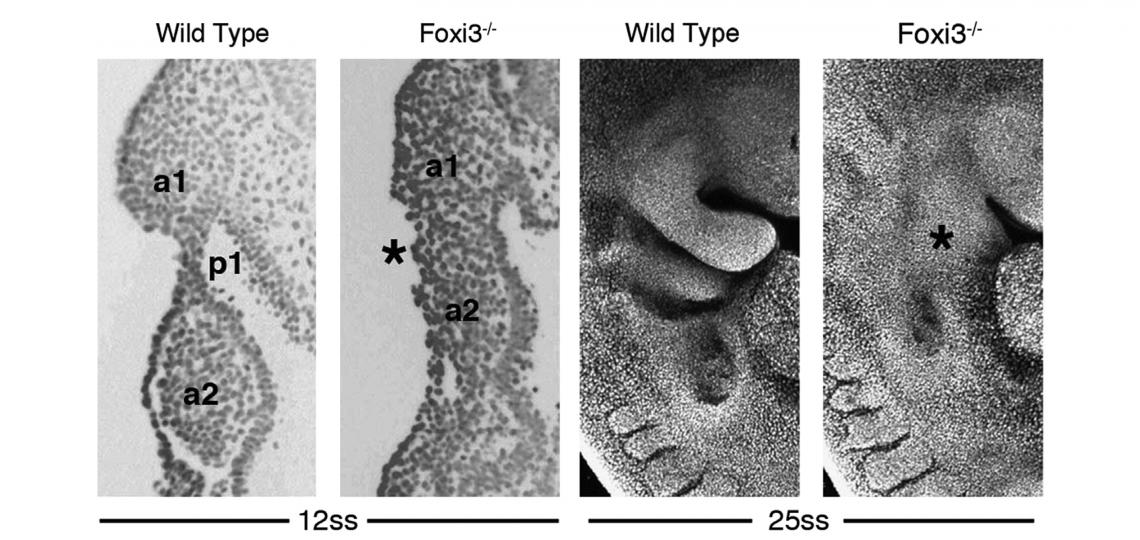
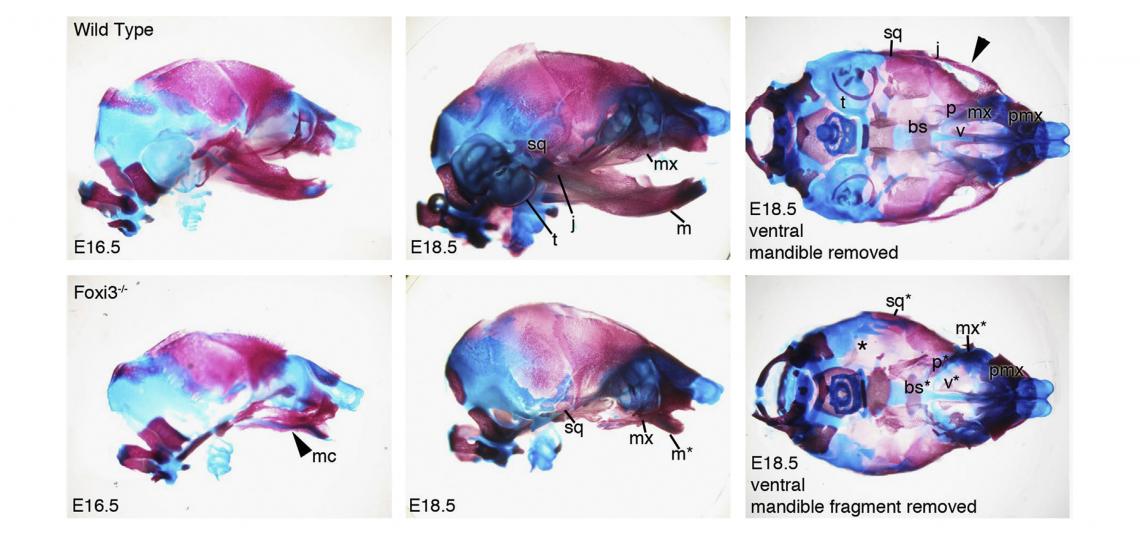
In the course of our work on the development of the inner ear, we identified and cloned the mouse Foxi3 transcription factor. Foxi3 is expressed very early in the inner ear anlagen and broadly in early pharyngeal endoderm and ectoderm. Foxi3 mutant mice generated in our lab completely lack all traces of the inner ear, but also show remarkably severe craniofacial defects. Foxi3 mice die between embryonic day 14 and birth, and lack all pharyngeal arch derivatives with the exception of the maxilla. Our data suggest that arches initially form in Foxi3 mutants, but rapidly degenerate. The early expression of Foxi3 in arch ectoderm and endoderm, together with the fact that Foxi3 mutants have some of the most severe craniofacial defects of any mouse mutant examined to date, suggest it is playing a very important role in the development and patterning of the pharyngeal arches. We recently showed it regulates the production of Fgf8 in pharyngeal ectoderm, which acts as a survival factor for migrating neural crest cells
Foxi3 is also expressed in non-sensory placodes that form hair, tooth and whisker follicles. Foxi3 is mutated in canine ectodermal dysplasia, a condition found in three breeds of hairless dogs (The Mexican hairless dog or Xolo, the Peruvian hairless dog and the Chinese crested dog). We generated Foxi3 conditional mice and are collaborating with groups in Switzerland and Finland to understand the role of Foxi3 in tooth and hair development. Interestingly, the dog mutation present in these three breeds causes a frame-shift mutation early in the gene that leads to the production of a largely novel protein which we think acts as a dominant neomorph. We are in the process of creating “canine-ized” mice carrying wild type and mutant dog Foxi3 genes to understand the function of this mutant protein.
Shirokova, V., Jussila, M., Biggs, L.C., Ohyama, T., Groves, A.K. and Mikkola, M.L. (2016). Foxi3 deficiency compromises hair follicle stem cell specification and activation. Stem Cells 10.1002/stem.2363
Jussila, M., Aalto, A., Sanz Navarro, M., Shirokova, V., Kallonen, A., Ohyama, T., Groves, A.K., Mikkola, M.L. and Thesleff, I. (2015). Suppression of epithelial differentiation by Foxi3 is essential for molar crown patterning. Development 142, 3954-3963.
Tassano, E., Jagannathan, V., Drögemüller, C., Leoni, M., Hytönen, M., Severino, M., Gimelli, S., Cuoco, C., Di Rocco, M., Sanio, K., Groves, A.K., Leeb, T and Gimelli, G. (2015). Congenital aural atresia associated with agenesis of internal carotid artery in a girl with a FOXI3 deletion. Am. J. Med Genetics 167A, 537-544.
Edlund, R.K., Ohyama, T., Kantarci, H., Riley, B.B. and Groves, A.K. (2014). Foxi transcription factors promote pharyngeal arch development by regulating the formation of FGF signaling centers. Developmental Biology 390, 1-13.