Indications For Use
PR belongs to a superfamily of nuclear hormone receptors. ER induces PR expression, and therefore PR status serves as an indicator of an intact ER pathway. There are two known isoforms of PR: PR-A and PR-B. The current assays in clinical breast cancer measure both isoforms. Nearly all the laboratories today measure PR by immunohistochemistry.
PR is expressed in about 60 percent of invasive breast cancers (1, 2). It is a weak prognostic factor by itself but a modest predictive factor that adds to the predictive value of ER for response to endocrine therapies, both in adjuvant and metastatic settings (3). The primary indication to assess PR in breast cancer is to predict response to hormonal therapies, such as tamoxifen, other selective estrogen receptor modulators (SERMs), aromatase inhibitors, etc. The College of American Pathologists (CAP) and American Society of Clinical Oncology (ASCO) have recommended that PR should be measured in all primary breast cancers using validated biochemical or immunohistochemical methods (4, 5).
Scoring/Interpretation
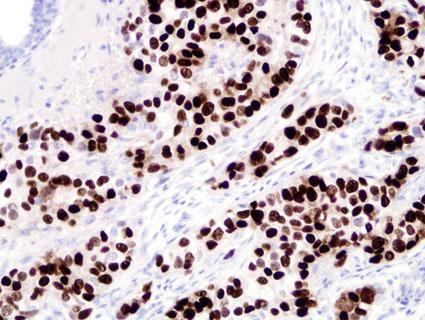
PR immunostaining is scored in our laboratory using the “Allred score” which is a microscopic method conveying the estimated proportion and intensity of positive tumor cells (range from 0-8). This scoring system was developed in our laboratory over a decade ago and has been used and validated for several markers, including, ER, PR, HER-2/neu and p53. Based on two large clinical studies using this scoring system and an assay based on antibody 1294, our laboratory has determined that "3" (corresponding to 1-10 percent positive cells) the optimum score for defining PR-positive breast cancer (6, 7). See detailed staining procedure.
Selected Bibliography
Elledge RM, Allred DC. Clinical aspects of estrogen and progesterone receptors. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the breast. Philadelphia: Lippincott Williams & Wilkins; 2004. p. 603-17.
Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 1998;11(2):155-68.
Tamoxifen for early breast breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet 1998;351:1451-67.
1997 update of recommendations for the use of tumor markers in breast and colorectal cancer. Adopted on November 7, 1997 by the American Society of Clinical Oncology. J Clin Oncol 1998;16(2):793-5.
Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark GM, et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000;124(7):966-78.
Love RR, Duc NB, Allred DC, Binh NC, Dinh NV, Kha NN, et al. Oophorectomy and tamoxifen adjuvant therapy in premenopausal Vietnamese and Chinese women with operable breast cancer. J Clin Oncol 2002;20(10):2559-66.
Mohsin SK, Weiss H, Havighurst T, Clark GM, Berardo M, Roanh LD, To TV, Zhang Q, Love RR, Allred DC. Progesterone receptor by immunohistochemistry and clinical outcome in breast cancer: a validation study. Modern Pathol 2004;17:1545-54.








