The Agent
Nearly everyone has experienced the fever, aches, and other symptoms of seasonal flu that afflicts 5 – 20 percent of Americans each year. Although these yearly flu epidemics can be fatal in some people, such as the elderly, young children, and people with certain underlying heath conditions, flu is generally not a life-threatening disease in healthy individuals.
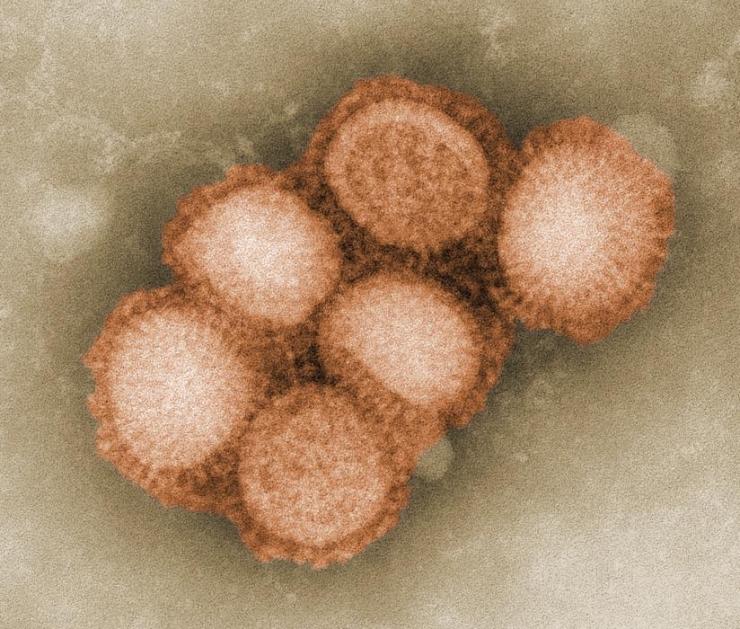
Flu, or influenza, is a contagious respiratory illness that spreads from person to person through the air via coughs or sneezes or through contact with infected surfaces. It is caused by a group of continuously changing viruses called influenza viruses.
Influenza viruses change easily and often, they are unpredictable, and they can be deadly. It is always a great concern when a new flu virus emerges, because the general population does not have immunity and almost everyone is susceptible to infection and disease.
Every few decades or so, a new version of the influenza virus emerges in the human population that causes a serious global outbreak of disease called a pandemic. Pandemics are associated with widespread illness - and sometimes death - even in otherwise healthy people. These outbreaks can also lead to social disruption and economic loss.
About a decade ago, scientists and public health officials feared that we might be on the brink of a pandemic caused by the so-called avian or bird H5N1 flu that began circulating among poultry, ducks, and geese in Asia and spread to Europe and Africa. To date, the avian flu virus has not acquired to ability to spread easily from person to person – a necessary step in order for a virus to cause a pandemic.
In the spring of 2009, a different influenza virus - one that had never been seen before - suddenly appeared. The novel virus, commonly called swine flu, is named influenza A (H1N1). Unlike the avian H5N1 flu, the H1N1 swine flu is capable of being transmitted easily from person to person. Fortunately, however, H1N1 is far less deadly than the H5N1 virus. In only a few short weeks after emerging in North America, the new H1N1 virus reached around the world. As a result of the rapid, global spread of H1N1, the first pandemic of the 21st century was declared in June of 2009.
Although the 2009 H1N1 pandemic did not turn out to be as deadly as initially feared, the next pandemic flu virus could emerge at any time, and we must remain vigilant. Hopefully, the knowledge gained in response to the H5N1 and 2009 H1N1 outbreaks, and continued research to more completely understand influenza virus, as well as improvements in vaccine and drug development, will enable us to minimize the effects of future influenza outbreaks.
Different Types of Influenza Virus
There are three different types of influenza virus – A, B, and C. Type A viruses infect humans and several types of animals, including birds, pigs, and horses. Type B influenza is normally found only in humans, and type C is mostly found in humans, but has also been found in pigs and dogs.
Influenza pandemics are caused by type A viruses, and therefore these are the most feared type of influenza virus; neither types B or C have caused pandemics.
Type A influenza is classified into subtypes depending on which versions of two different proteins are present on the surface of the virus. These proteins are called hemagglutinin (HA) and neuraminidase (NA). There are 17 different versions of HA and 10 different versions of NA. So, for example, a virus with version 1 of the HA protein and version 2 of the NA protein would be called influenza A subtype H1N2 (A H1N2, for short).
The influenza A subtypes are further classified into strains, and the names of the virus strains include the place where the strain was first found and the year of discovery. Therefore, an H1N1 strain isolated in California in 2009 is referred to as A/California/07/2009 (H1N1).
Although many different combinations of the HA and NA proteins are possible, viruses with only a few of the possible combinations circulate through the human population at any given time. Currently, subtypes H1N1 and H3N2 are in general circulation in people. Other combinations circulate in animals, such as the H5N1 virus found in birds. The subtypes that exist within a population change over time. For example, the H2N2 subtype, which infected people between 1957 and 1968, is no longer found in humans.
What Influenza Viruses Are Made of
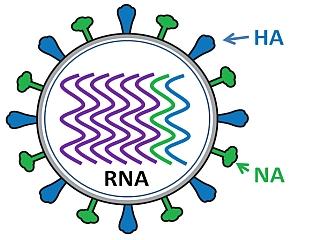
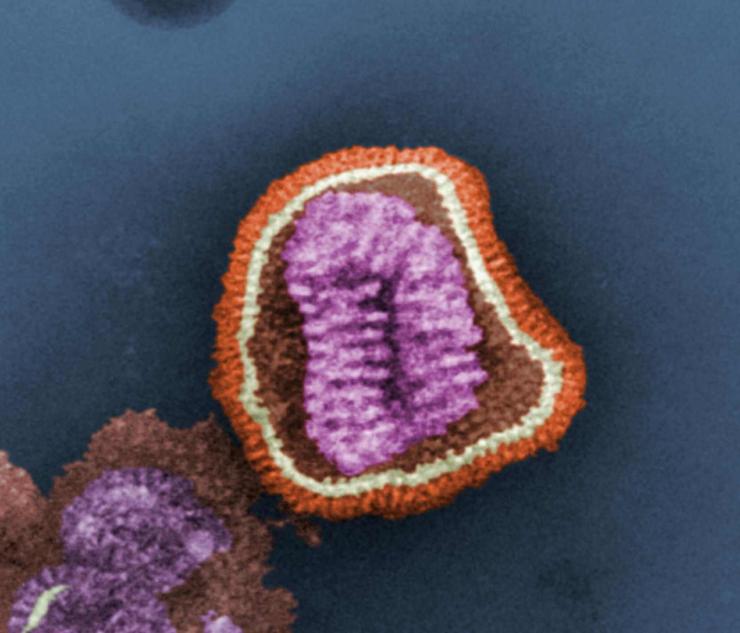
Influenza virus has a rounded shape (although it can be elongated or irregularly shaped) and has a layer of spikes on the outside.
There are two different kinds of spikes, each made of a different protein – one is the hemagglutinin (HA) protein and the other is the neuraminidase (NA) protein.
The HA protein allows the virus to stick to a cell, so that it can enter into a host cell and start the infection process (all viruses need to enter cells in order to make more copies of themselves).
The NA protein is needed for the virus to exit the host cell, so that the new viruses that were made inside the host cell can go on to infect more cells. Because these proteins are present on the surface of the virus, they are “visible” to the human immune system.
Inside the layer of spikes, there are eight pieces, or segments, of RNA that contain the genetic information for making new copies of the virus. Each of these segments contains the instructions to make one or more proteins of the virus. So for example, segment 4 contains the instructions to make the HA protein, and segment 6 contains the instructions to make the NA protein (the segments are numbered in size order, with 1 being the largest).
When new viruses are made inside the host cell, all eight segments need to be assembled into a new virus particle, so that each virus has the complete set of instructions for making a new virus. The danger occurs when there are two different subtypes of influenza A inside the same cell, and the segments become mixed to create a new virus.
How Influenza Viruses Change
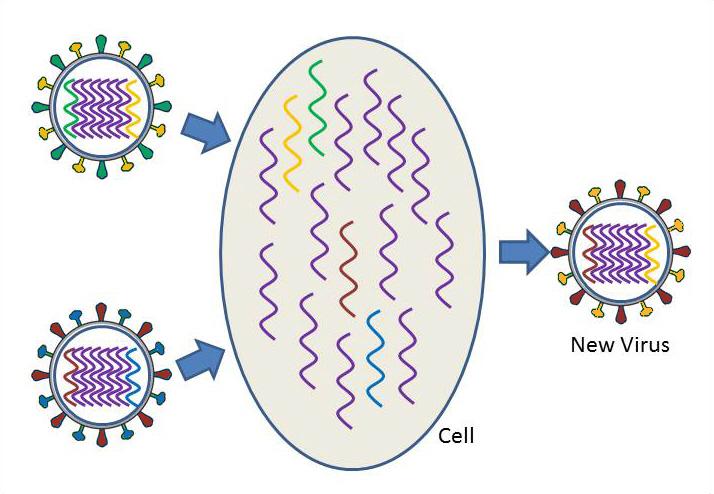
Influenza virus is one of the most changeable viruses known. There are two ways that influenza virus changes – these are called drift and shift.
Drifting, or antigenic drift, is a gradual, continuous change that occurs when the virus makes small “mistakes” when copying its genetic information. This can result in a slight difference in the HA or NA proteins. Although the changes may be small, they may be significant enough so that the human immune system will no longer recognize and defend against the altered proteins. This is why you can repeatedly get the flu and why flu vaccines must be administered each year to combat the current circulating strains of the virus.
Shifting, or antigenic shift, is an abrupt, major change in the virus, which produces a new combination of the HA and NA proteins. These new influenza virus subtypes have not been seen in humans (or at least not for a very long time), and because they are so different from existing influenza viruses, people have very little protection against them. When this happens, and the newly created subtype can be transmitted easily from one person to another, a pandemic could occur.
Virus shift can take place when a person or animal is infected with two different subtypes of influenza. Take the case, for example, where there are two different subtypes of influenza circulating at the same time, one in humans and one in ducks. The human subtype is able to infect humans and pigs, but not ducks, while the duck subtype is able to infect ducks and pigs, but not humans. When a pig becomes infected with both the human and duck influenza subtypes at the same time, the segments of both viruses are scrambled or reassorted. inside an infected pig cell. As a result, a human virus particle could assemble that contains the duck HA segment instead of the human HA segment. A new virus subtype has been created. This new subtype could infect humans, but because it has the new duck version of the HA protein, the human immune system would not be able to defend an infected person against the new virus subtype. The virus could continue to change to allow it to spread more easily in its new host, and widespread illness and death could result.
Virus shift can also occur when an avian strain becomes adapted to humans, so that the avian virus is easily transmitted from person to person. In this case, the avian strain jumps directly from birds to humans, without mixing or reassortment of the genetic material of influenza strains from different species.
Influenza Epidemics and Pandemics
Influenza epidemics, also known as seasonal flu, occur annually and are the most common emerging infection among humans. These epidemics have major medical impacts, but they are generally not fatal except in certain groups such as the elderly.
Pandemics, on the other hand, happen once every few decades on average. They occur when a new subtype of influenza A arises that has either never circulated in the human population or has not circulated for a very long time (so that most people do not have immunity against the virus). The new subtype often causes serious illness and death, even among healthy individuals, and can spread easily through the human population.
There were three influenza pandemics in the 20th century – the “Spanish” flu of 1918-19, the “Asian” flu of 1957-58, and the “Hong Kong” flu of 1968-69. The 1918 flu, caused by a strain of H1N1, was by far the most deadly. More than 500,000 people died in the United States as a result of the Spanish flu, and up to 50 million people may have died worldwide. Nearly half of those of those deaths were among young, otherwise healthy individuals. The 1957 pandemic was due to a new H2N2 strain of influenza virus that caused the deaths of two million people, while the 1968 pandemic resulted from an H3N2 strain that killed one million people.
One pandemic has occurred so far in the 21st century. This was due to the novel swine-origin H1N1 virus which emerged in 2009.
The WHO established a six phase pandemic alert system in 2005 in response to the potential threat of the H5N1 avian influenza virus. The alert system is based on the geographic spread of the virus, not necessarily the severity of disease caused by the virus. Although a disease may be “moderate” in severity, during widespread outbreaks, declaration of a pandemic is beneficial because it accelerates the vaccine production and prompts governments to take extra measures to contain the virus. Travel and trade bans may be implemented in some cases, although if the disease is already widespread, these may not be considered effective.
Prior to the emergence of the 2009 H1N1 virus, the alert level stood at Phase 3 based on the circulation of the H5N1 virus. On April 27, 2009, after the H1N1 flu virus was recognized to be passing from person to person in Mexico, the alert level was raised to Phase 4. Two days later, on April 29, the WHO again increased the alert level, this time to Phase 5, reflecting the sustained transmission of the novel H1N1 virus in the United States. As H1N1 continued to spread worldwide and infect people in over 70 countries, the WHO raised the alert to Phase 6 – the highest level - on June 11, 2009. Over the next few months, H1N1 spread to more than 200 countries and territories worldwide. The Phase 6 alert of the 2009 H1N1 pandemic was declared by the WHO to have ended on Aug. 10, 2010.
Avian Flu
Influenza naturally infects wild birds all around the world, although they usually do not become ill. The virus is very contagious, however, and it can become a problem when the virus is transmitted to domesticated birds, such as chickens, ducks, or turkeys, because domesticated poultry can succumb to illness and death from influenza.
Humans generally do not become infected with avian flu. That is why news of humans contracting avian influenza during an outbreak of bird flu in poultry in 1997 in Hong Kong was alarming. It indicated that the virus had changed to allow it to directly infect humans. The virus that caused this particular outbreak is influenza A subtype H5N1.
Since 1997, H5N1 infections in birds have spread, initially throughout Asia. Then as birds traveled along their migratory routes, H5N1 dispersed to Russia and Europe, and later to countries in the Middle East and on the African continent.
Most human cases of H5N1 influenza have been traced to direct contact with infected poultry, but there have been a few cases of person-to-person transmission, particularly in clusters where multiple family members became infected.
One reason why avian H5N1 is not readily transmissible among people has to do with the hemagglutinin, or HA, protein of the virus that determines which cell type the virus can enter. As with other viruses, the influenza virus must attach to specific proteins called receptors on the outside of cells in order to gain entry into cells and cause an infection. Unlike human influenza viruses, which infect cells high in the respiratory tract, the H5N1 HA protein attaches to cells much lower in the respiratory track. The virus is so deep within the respiratory tract that it is not coughed up or sneezed out, and so it does not easily infect other people. If the HA protein of H5N1 were to mutate so that it could infect cells higher in the respiratory tract, then it would more likely be able to pass from person to person.
As of July 2015, there have been some 840 laboratory-confirmed cases of H5N1 infections in humans, in 16 different countries, and close to 450 deaths. The countries with the overall highest case numbers are Egypt, where almost all cases in 2015 have occurred, followed by Indonesia and Vietnam.
H5N1 continues to circulate in poultry, and small and sporadic clusters of human infections are still occurring. However, H5N1 currently does not transmit easily between people, so the risk of a large outbreak is low at this time.
Highly pathogenic H5 avian virus infections were first reported in birds in the United States in December 2014. Over approximately the next six months, more than 200 findings of infection with H5N2, H5N8, and H5N1 viruses were confirmed, mostly in poultry including backyard and commercial flocks. More than 40 million birds in 20 states were either infected or exposed. No human infections by these H5 viruses have been reported in the United States, but their presence in birds makes it more likely than human H5 infections could occur in the United States. Individuals having close contact with live infected poultry or surfaces contaminated with the avian influenza viruses are at highest risk of infection in places where the viruses circulate. There have been no reports of infection occurring from eating properly cooked poultry.
In addition to the H5 viral subtypes, other avian influenza strains have occasionally infected humans in recent years. These include the H7N2 strain which infected two individuals in the eastern United States in 2002 and 2003, and the H9N2 strain which has caused illness in several people in Asia in 1999 and 2003.
In March of 2013, a new subtype of avian influenza was found to infect humans. Influenza A (H7N9) had previously been detected in birds, but this particular variant had never been seen before in humans or animals. The initial wave of H7N9 infections occurred in the spring of 2013 in China, followed by a larger, second wave in the first half of 2014 in China and a few neighboring countries. As of February 2015, approximately 570 cases and 210 deaths have been reported to the WHO, mostly in China. People in the majority of cases had exposure to infected poultry or contaminated environments. The H7N9 virus causes a severe respiratory illness in most infected people, but it currently does not appear to spread easily from person to person.
Swine Flu
Swine influenza, or swine flu, is a very contagious respiratory disease of pigs. Although pigs become ill, they generally do not die from swine flu viruses.
In April of 2009, an influenza virus originating in swine was discovered to be capable of infecting humans and spreading from person to person. The new virus is named influenza A (H1N1), although it is commonly referred to as swine flu. Although it is called swine flu, the new H1N1 virus is transmitted from person to person, and not through contact with pigs or pork products.
The new H1N1 virus is made up of a novel combination of segments from four different influenza virus strains - a Eurasian swine virus, a North American swine virus, and avian and human influenza virus segments. Reassortment of segments from these different viruses produced a unique virus that had not been seen before by the human population. When novel viruses like this emerge, natural immunity is usually limited or nonexistent in humans.
The H1N1 influenza virus outbreak originated in Mexico in early 2009, and then spread rapidly throughout North America. Within a few weeks, the novel swine-origin H1N1 virus extended its reach around the globe. In June 2009, as a result of the global spread of the H1N1 virus, the WHO issued its first pandemic declaration of the 21st century - the first since the flu pandemic of 1968. The pandemic declaration acknowledged the inability to contain the virus and recognized its inevitable further spread within affected countries and into new countries. The new H1N1 virus became the dominant influenza strain in most parts of the world, including the United States.
Like other influenza pandemics, the 2009 H1N1 outbreak occurred in waves. The first wave took place in the spring of 2009, with a second wave commencing in late August as children and college students returned to classes. The outbreak peaked in October of 2009, with flu activity reported in all 50 states, as well as numerous other countries and territories. By January 2010, flu activity had returned to below baseline levels.
The H1N1 virus continues to circulate at low levels, but it is no longer the dominant influenza strain, and its behavior more closely resembles a seasonal influenza virus than a pandemic flu.
From the time the outbreak began in April 2009 through April 2010, the CDC estimated that about 60 million Americans became infected with the H1N1 virus, 265,000 Americans were hospitalized and 12,000 deaths occurred as a consequence of the 2009 H1N1 flu. The highest hospitalization rates occurred in young children. Exact numbers are not known due to the widespread nature of the outbreak and because most patients, especially those with mild cases, were not tested. The large majority of infections in the United States and most other countries were mild, although pregnant women and individuals with certain underlying medical conditions had an increased risk of severe and fatal illness.
There were some differences between the pandemic H1N1 flu and regular, seasonal flu. First, the H1N1 flu continued to spread during the summer months, which is uncommon for seasonal flu. Second, a much larger percentage of H1N1 patients exhibited symptoms of vomiting and diarrhea than is common with regular seasonal flu. There were also more reports of severe respiratory disease, especially in young and otherwise healthy people, infected with the new H1N1 virus than with seasonal flu viruses.
Significantly, the majority of cases of H1N1 infection, including severe and fatal cases, occurred in young and otherwise healthy individuals generally between the ages of 5 and 50, with relatively few deaths among the elderly. This is in contrast to the situation with seasonal flu which primarily afflicts the very young and the elderly, and where 90 percent of severe and lethal cases occur in people over the age of 65. Deaths among the elderly accounted for only 11 percent of H1N1 deaths.
Fortunately, the 2009 H1N1 flu was sensitive to two antiviral drugs used to treat influenza - Tamiflu® (oseltamivir) and Relenza® (zanamivir). The drugs act by inhibiting the essential neuraminidase protein (the “N” protein in the naming system). Proper use of these drugs can shorten the duration and lessen the severity of the sickness and reduce the chance of spreading the disease. The drugs reduce the risk of pneumonia - a major cause of death from influenza - and the need for hospitalization. To be most effective, the antiviral drugs should be administered as soon as possible after the onset of symptoms.
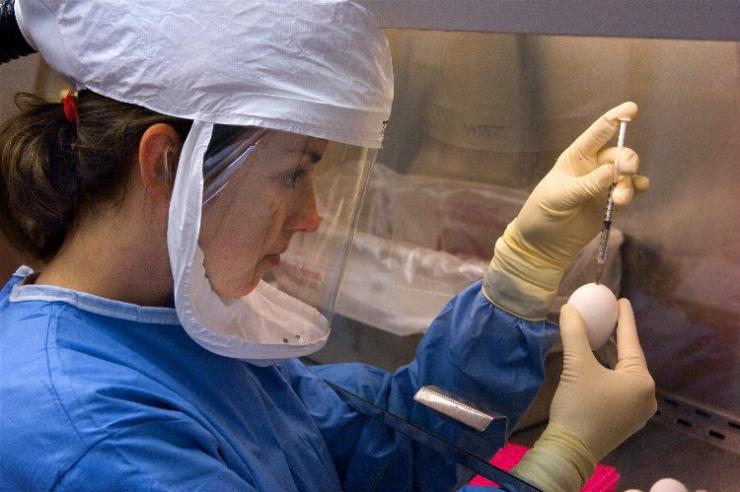
A vaccine to protect against the H1N1 virus was developed, tested, and approved and became available in October 2009. Due to the fact that the virus used to prepare the vaccine grew more slowly than most seasonal flu viruses do, production of the vaccine lagged and widespread distribution of the vaccine occurred later than anticipated. Priority for the vaccine was initially given to health care and emergency workers and individuals at high risk for severe disease, but by the winter of 2009-2010 availability was extended to the general population. Later, some doses went unused.
Although some had concerns about the safety of the H1N1 vaccine, flu vaccines have a very good safety profile. While mild side effects, such as soreness at the site of injection, aches, and low-grade fever, may occur as a result of receiving a flu shot, it is not possible to get the flu (H1N1 or seasonal) from the vaccine. The flu shot, or inactivated vaccine, is made from only a portion of the virus – a purified protein that makes our immune system develop protection. Likewise, the nasal spray version of the flu vaccine contains attenuated or weakened virus that is not able to cause the flu. Given the potential serious health outcomes from the flu, especially for high-risk population groups, the benefits of vaccination as the best way to prevent influenza infection and its complications far outweigh the risk of relatively minor side effects from the vaccination.
The Problem
Historically, influenza pandemics arise about three to four times each century. The most recent pandemic, and the first of the 21st century, occurred in 2009, some 40 years after the previous pandemic. The 2009 H1N1 flu, commonly known as swine flu, spread around the globe faster than any virus in history, largely due to air travel.
Pandemic flu strains are of deep concern because there is no or only limited natural immunity to novel flu strains, and therefore nearly everyone is susceptible to infection. A high percentage of the population could become ill at any one time and overwhelm public health systems, and a large number of deaths could occur.
We were very fortunate in the case of the 2009 H1N1 pandemic. Most people suffered only a mild illness. H1N1 was not an especially virulent virus. Further, the virus remained stable and did not mutate to a more deadly form or to a drug resistant form.
Other influenza strains have been far more lethal. The flu strain that caused the 1918 pandemic began in the spring as a mild flu, but returned as a more deadly version in the fall and winter of that year and went on to infect about one-third of the world’s population and kill an estimated 50 million people. The avian H5N1 virus, that is still circulating, has a mortality rate of near 60%, although it does not easily pass from person to person.
Currently, there is concern about the new avian H7N9 virus. Most patients have experienced severe respiratory illness, with about one-third of the cases resulting in death. Although the virus does not appear to pass easily from person to person, there is always the worry that it could mutate into a form that is more transmissible.
There are drugs that are effective against influenza, but the possibility that a virus could acquire resistance to the drugs is a serious issue. There are four different antiviral drugs, of two different classes, that are effective against influenza. However, influenza viruses can and do develop resistance to these drugs - as one of the main circulating seasonal viruses did during a recent flu season - so that the drugs can no longer be used to treat or prevent infections. There is a need to develop additional drugs that can prevent or alleviate flu symptoms.
Vaccines can be developed to protect humans from influenza viruses. However, as was strikingly obvious during the 2009 H1N1 pandemic, vaccine production takes many months. By the time a vaccine was developed, tested, produced, and distributed, many individuals had already been infected. Clearly, a more rapid method of vaccine development is needed. The goal of developing a universal flu vaccine, one that would provide durable protection against multiple flu strains, remains a challenging feat.
The greatest fear is that a new pandemic influenza virus could emerge that could pass from person to person as easily as the H1N1 virus, but be as deadly as the H5N1 virus. Additional concerns are that an influenza virus could mutate into a form that would be resistant to anti-influenza drugs, such as Tamiflu, or that the virus could change so that a vaccine no longer afforded protection.
Even though the 2009 H1N1 pandemic was relatively mild, knowing how lethal and unpredictable influenza viruses can be, we must continue to remain alert and prepare for future pandemics.
The Research
Investigators in the Department of Molecular Virology and Microbiology (MVM) have been studying influenza for several decades, with an Influenza Research Center first established in 1974. A major focus of the work is directed towards the development and testing of influenza vaccines to find the most effective vaccination dosages, methods, and strategies to protect the population against this deadly disease. Other projects involve studying the structure and function of important influenza proteins.
Research is ongoing on both epidemic influenza (also referred to as seasonal or interpandemic influenza) and pandemic influenza. Epidemic influenza occurs annually and is attributable to minor changes in genes that encode proteins on the surface of circulating influenza viruses. Pandemic influenza occurs when more significant changes in the influenza A virus arise as a result of the acquisition of genes from influenza viruses of other animal species by a human virus strain, thus creating a novel virus. The latter carries a greater risk for the human population.
Notably, the department is home to the BCM Vaccine and Treatment Evaluation Unit (VTEU), one of nine federally funded centers in the nation established by the National Institute of Allergy and Infectious Diseases. It was previously led by Dr. Wendy Keitel and is currently under the direction of Dr. Hana El-Sahly. The VTEU network conducts clinical trials that evaluate vaccines and treatments for a wide array of infectious diseases. An important strength of this established network is that it is able to efficiently and safely test new vaccines within a rapid time frame.
The VTEU research group in the department has been involved in important studies that led to the licensure of live attenuated and high dose inactivated influenza virus vaccines. They have tested vaccines to seasonal influenza and they have performed many studies evaluating vaccines targeting pandemic influenza, including the swine-origin H1N1, and the H5N1, H9N2, and H7N9 viruses, among others. They have evaluated methods to improve vaccine immunogenicity, including delivery of vaccine by different routes of administration, different dosages, and with different adjuvant preparations.
Researchers involved in these studies include Drs. Robert Atmar, Robert Couch, Hana El Sahly, Paul Glezen, Wendy Keitel, Innocent Mbawuike, Flor Munoz-Rivas, Shital Patel, and Pedro (Tony) Piedra. Their hope is that the results of these studies will identify the optimal and most effective dosages of vaccine to protect the public from seasonal influenza, as well as from a possible influenza pandemic.
Epidemic Influenza
MVM investigators would like to better understand epidemic influenza (seasonal flu) infections, disease, and vaccines with the goal of developing ways to better control these epidemics.
Towards this goal, they are working on developing new improved vaccines against epidemic influenza strains and are trying to understand how the immune systems of different people respond to the influenza virus and influenza vaccines.
Specifically, to gain this knowledge scientists are engaged in the following projects:
- Developing new vaccines for induction of humoral and cell-mediated immune responses against influenza viruses that can prevent or modify infections.
- Identifying the optimal way to induce mucosal immune responses to influenza viruses that can increase resistance to infection at the site where infection initially occurs.
- Searching human genes for single nucleotide polymorphisms that determine the pattern and magnitude of immune response to influenza virus or provide an explanation for illness and its severity.
- Determining the role of immune responses directed toward the different proteins of influenza, including new candidates, for a beneficial role.
- Performing clinical trials of new and experimental vaccines as part of a program for development of improved influenza vaccines.
- Developing improved methods for measuring immune function in humans.
In addition, MVM researchers with the VTEU have been evaluating the safety and immunogenicity of seasonal influenza vaccine in pregnant women. Because pregnant women are at higher risk for serious complications from the flu, it is important to develop strategies to protect these women from seasonal and pandemic influenza. The clinical trial includes up to 200 women recruited from nine sites across the nation and is headed by Dr. Shital Patel. It is one of the few studies that will evaluate antibody responses in pregnant women following vaccination. Evaluating the safety of seasonal inactivated influenza vaccine will yield vital information in anticipation of the need to test novel vaccines against possible future pandemic strains, in pregnant women.
Scientists are also conducting a study (in collaboration with Kelsey-Seybold Clinics) to determine the effectiveness of an inactivated influenza vaccine in protecting pregnant women and whether these immunized women can pass immunity against influenza to their infants, so that newborns would be protected from influenza during their first few months of life.
Another approach to protect against influenza epidemics is called herd immunity. The idea is to vaccinate a large percentage of school-age children to limit the spread of influenza without needing to vaccinate a larger percentage of the general population. The reasoning behind this idea is that school-age children are often the source of infection and pass the virus onto their friends, teachers, and family members. So preventing children from spreading influenza through large-scale vaccination of this group should protect the rest of the “herd” from influenza infection, even those who haven’t been vaccinated. This might be especially helpful to the elderly population who are at higher risk from influenza-related complications and whose immune systems may not mount as effective a response to influenza as younger individuals. Another advantage to this approach is that it might be possible to achieve high community protection from influenza with a limiting amount of vaccine.
Dr. Pedro (Tony) Piedra and colleagues are testing herd immunization in school-aged children in central Texas. In their initial study, they found that vaccination of 12 to 15 percent of children in selected communities resulted in an indirect protection to influenza infection in 8 to 18 percent of the adults in these communities. They are currently conducting a larger, school-based vaccination program with the goal of immunizing 50 percent of the children, and they will determine how effective this level of immunization is in preventing infection in adults. Dr. Piedra and co-workers want to know how many children need to be vaccinated in order to protect the adult population from influenza infection, and they would like to use this approach to control the spread of epidemic influenza. They also hope to use this approach as a model for combating pandemic influenza and bioterrorism.
Dr. Piedra has also investigated the effects of oseltamivir (commonly known as Tamiflu) on influenza-related complications in children with chronic medical conditions. Patients with underlying medical conditions are at higher risk of complications from both seasonal and pandemic flu. Dr. Piedra and his colleagues found that children with chronic medical conditions benefit from the use of Tamiflu if it is prescribed early in the disease process. Children and adolescents between the ages of 1 and 17 who were at high risk of influenza complications showed significant reductions in the risks of respiratory illnesses other than pneumonia, reduced risk of otitis media (a middle ear infection), and fewer hospitalizations in the 14 days after influenza diagnosis.
Pandemic Influenza
The most effective way to prevent the widespread infection and high mortality rate that a new influenza virus could inflict upon the human population would be to vaccinate people, so that the human immune system would be prepared to fight off an infection.
MVM investigators are trying to identify the best way to prime the human immune system to defend against flu strains that could cause a pandemic.
Researchers have been testing vaccines against H1N1, H5N1, H7N9, and other potential pandemic flu strains and analyzing the immune responses of different people to the vaccines.
Specific projects include the following:
- Studies of vaccines against different potential pandemic influenza virus strains (H5N1, H9N2, and H7N9)
- Studies of pandemic influenza vaccines given by different routes (intramuscularly, intradermally) and in different dosages
- Studies to determine whether immune responses are improved when a pandemic influenza virus vaccine strain is combined with an adjuvant
- Studies of pandemic influenza vaccines in different age groups
- Developing methods for measuring immune responses to these vaccines
Members of the Department were part of the national effort to prepare a vaccine against H1N1 influenza and test candidate vaccines. MVM researchers within the VTEU network evaluated the safety and effectiveness of H1N1 candidate vaccines produced by two different manufacturers (Sanofi Pasteur and CSL Biotherapies). Several different parameters were tested: the number of doses required (one or two), different dosage amounts (15 or 30 micrograms), and different age groups (18 to 64 years old, age 65 and older, and healthy children). The goal was to determine the reactions and antibody protection responses following immunization with experimental influenza H1N1 vaccine when given with seasonal influenza vaccine. The trial enrolled healthy adults, and a similar trial was conducted with children aged 6 months to 17 years. Study participants received a single strength of the 2009 H1N1 vaccine given in two doses along with the seasonal flu vaccine given either before, during, or after the time that they were inoculated with the H1N1 vaccine.
Scientists in the department also examined ways to optimize the collection of samples and testing for infection, analyzing immune responses, and working on epidemiological, pathogenesis, and treatment studies of the virus. They worked on developing a method to collect samples and isolate viruses so that they could assess the viruses and the immune responses against them. They enrolled patients with confirmed cases of H1N1 infection and collected nasal, throat and/or blood samples. Researchers used these samples to isolate the virus for further characterization and study how the immune system responds. These samples were banked and shared with researchers around the country. The goal of this study was to help guide the process of vaccine development and to give scientists an idea of the response to antiviral chemotherapy and changes of the virus over time.
MVM researchers have been involved in preparing assays used to detect the virus and evaluate immune responses. The Respiratory Virus Diagnostic Research laboratory supports clinical trials on the epidemiology, immunology, pathogenesis, and vaccine prevention of important human respiratory pathogens and houses a cell culture lab for virus isolation and a polymerase chain reaction (PCR) lab for respiratory virus identification. Under the direction of Dr. Pedro Piedra, the lab tests for most of the known respiratory viral pathogens and expanded its capabilities to include the swine-origin influenza A/H1N1 virus. In addition, Dr. Robert Couch set up serologic assays for evaluation of immune responses.
Studies conducted by MVM researchers helped guide public health officials in determining the best course of action in dealing with the 2009 H1N1 outbreak. They monitored vaccine recommendations made by the CDC and made suggestions. They kept the general public informed through local and national media outlets. The scientists continue to study the H1N1 virus to gain a deeper understanding of the virus itself, its interactions with the immune system, and responses to the H1N1 vaccine.
Additionally, researchers within the VTEU have been working on other new influenza strains that have pandemic potential including the new avian H7N9 virus and investigating vaccine strategies. This work will provide valuable information in responding to future influenza outbreaks.
In more recent work, Dr. Robert Atmar and his colleagues have been conducting a phase 2 clinical trial to test a candidate for a universal flu vaccine known as M-001. Unlike other flu vaccines, which consist of a whole inactivated flu virus or an attenuated live virus, the M-001 vaccine is comprised of nine epitopes, short stretches of viral protein, that are common to many different influenza strains. Because these regions are shared between different strains, it is hoped that the vaccine would trigger the immune system to respond to multiple strains. Adult healthy volunteers will receive the experimental vaccine or a placebo and will be monitored over time to evaluate their immune responses.
Structure and Function of Influenza Proteins
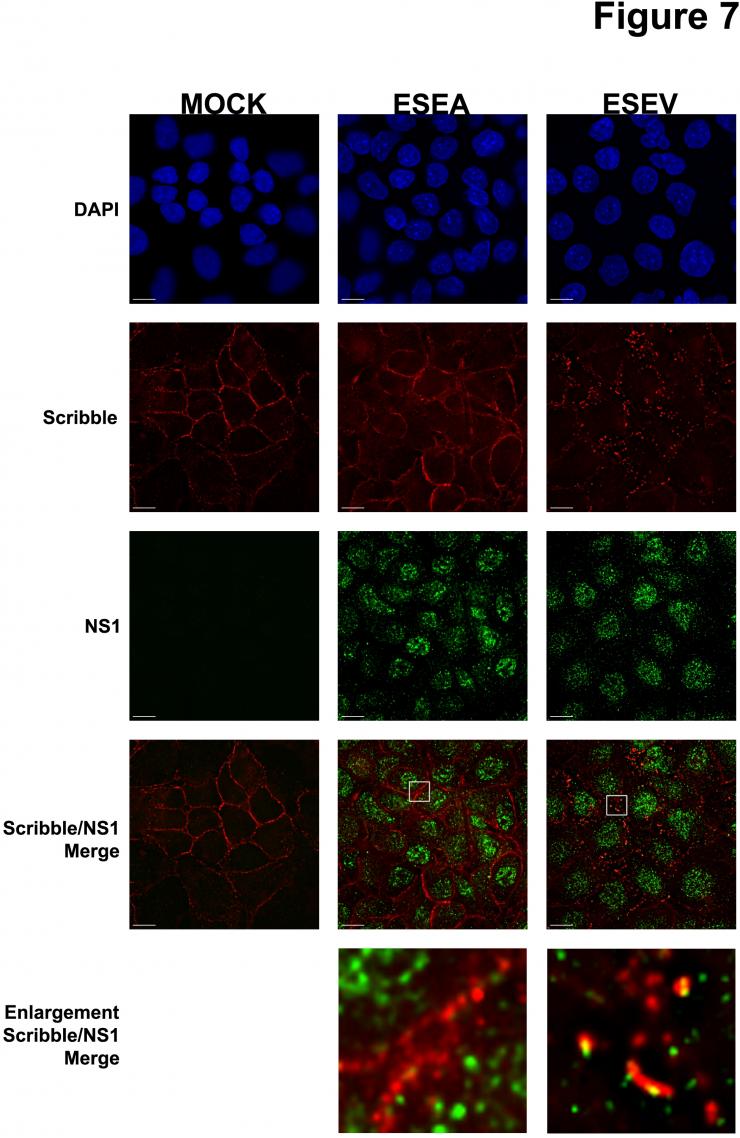
Dr. Andrew Rice and colleagues are studying an avian influenza virus protein called NS1 that has recently been shown to be associated with virulence. Proteins like NS1 that are involved in pathogenesis are important targets for novel antiviral therapeutics. The goal of this project is to identify cellular proteins that interact with NS1 and play a role in the pathogenesis of avian influenza virus infection. A critical feature of the avian NS1 protein is the presence of a protein domain at one end of the protein - the carboxyl terminus – that is termed the PDZ-Binding Motif, or PBM. The PBM is predicted to associate with a class of cellular proteins - termed PDZ proteins - that are typically involved in cell-cell contact, cellular polarity, and signaling pathways. It is notable that the NS1 protein of the virulent influenza viruses, such as H5N1, contains a PBM with the sequence ESEV, while other less virulent influenza viruses contain a different PBM sequence or lack this region entirely.
Dr. Rice and colleagues have identified a number of cellular targets of the NS1 PDZ-ligand domain: Dlg1, Scribble, MAGI-1, MAGI-2, and MAGI-3. Their current research involves the investigation of the functional significance of the interaction between NS1 with the ESEV PBM and these protein targets. Their results to date have shown that the ESEV PBM reduces apoptosis during infection and enhances the level of viral replication. They have also found that the ESEV PBM is involved in the disruption of cellular tight junctions during infection. A long-term goal of this project is to derive small molecules that can inhibit the interaction between the NS1 protein and its cellular PDZ protein targets, as such small molecules may be the basis for the development of novel therapeutics to treat avian influenza virus infection.
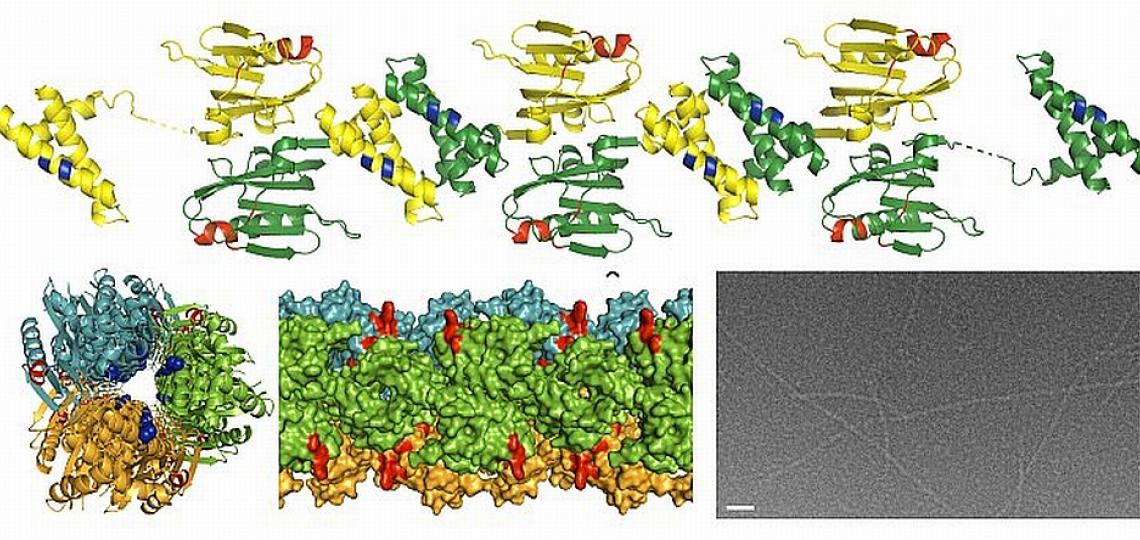
In a separate study, Dr. B.V. Venkataram Prasad and Zach Bornholdt, a graduate student in his laboratory, have determined the structure of a region of an important influenza protein called NS1. Their work may explain, in part, why the H5N1 virus causes such a severe and often fatal illness. NS1, a protein essential for influenza infection, antagonizes the cellular immune response and is thought to play a role in virulence. The lethal H5N1 strain has a different version of the NS1 protein than the NS1 protein of other strains of influenza. By knowing the structure of the NS1 protein, these investigators can surmise how variations in the H5N1 version of NS1 may alter its ability to interact with other molecules. They hypothesize that the mutations or changes in the H5N1 NS1 protein allow it to overcome the cellular immune response more effectively than the NS1 proteins of other strains of influenza. With detailed knowledge of the structure of the NS1 protein and how it interacts with other components of the cell, it will be easier to design drugs to specifically block these interactions and possibly disrupt the ability of the NS1 protein to interfere with the host’s protective immune response.
X-ray structure of the NS1 protein from a highly pathogenic H5N1 influenza virus. Provided by Dr. B.V.V. Prasad
For More Information
Seasonal Flu
- Seasonal flu information from the CDC
- Summary and maps of weekly seasonal flu activity from the CDC
- General flu information from the US Department of Health and Human Services
- Flu information from the National Institute of Allergy and Infectious Diseases
- US flu trend map from Google
- Flu vaccine advice from experts at BCM
Pandemic Flu
- Information about avian flu in humans from the WHO
- Information about swine flu in humans from the CDC
- Information about the 2009 H1N1 swine flu from the CDC
- Information about the 2009 H1N1 swine flu from the WHO
- Information about avian H5N1 flu in humans from the CDC
- Information about avian H7N9 flu in humans from the CDC
Glossary
Learn more about some of the technical terms found on this page by visiting our glossary of terms.
View a Flu Presentation
2009 H1N1 Influenza (Swine Flu) Pandemic (ppt)








 Credit
Credit