The Agent
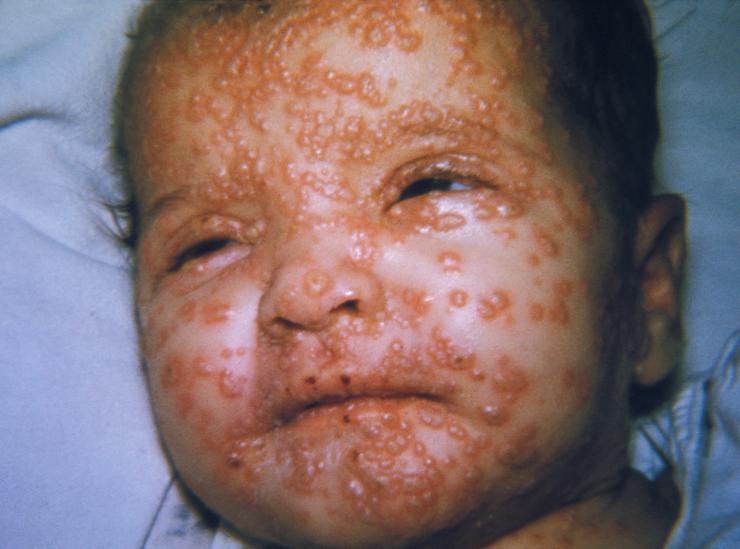
Smallpox, one of the biggest killers in history, is caused by a virus called variola. Variola causes a distinctive rash and is often lethal. The name variola comes from the Latin word for “spotted” and refers to the raised bumps that appear on the face and body of infected individuals. Although similar in name and in the formation of a rash, blisters, and scabs, variola belongs to a different virus family than the virus that causes the common childhood illness chicken pox. Variola is a member of the Poxvirus family of viruses. A close relative of variola within the Poxvirus family, called vaccinia, does not cause smallpox and is used as a vaccine for smallpox (in fact, the word vaccine comes from vaccinia virus). Vaccinia is also used in the laboratory to study this type of virus, because it is less hazardous to work with than the smallpox virus.
A virus related to variola called monkeypox virus recently made headlines when an outbreak occurred in the Midwestern region of the United States in 2003. This disease, also characterized by a rash and blisters, was the first monkeypox outbreak in the Western Hemisphere. Monkeypox virus sickened about 70 people. The cause was traced to prairie dogs that had been infected by imported African rodents at a pet distribution center. Fortunately, this disease was not as deadly as smallpox.
People generally become infected with the smallpox virus by breathing in virus droplets following exposure to infected individuals or by direct contact with infected fluids or contaminated objects. An unusual property of the smallpox virus is that it only infects humans and not animals and insects (this property was instrumental in the eradication of smallpox). After exposure to the virus, it usually takes one to two weeks before a person becomes ill and a rash and fever develop. At this point, the person is highly contagious and remains contagious until all scabs fall off after about three weeks. About 30% of infected people die from smallpox infection. People who recover from the infection are often left with permanent scars and sometimes blindness.
By some estimates, smallpox has been responsible for more deaths over the centuries than all other infectious diseases combined. A worldwide immunization program was instituted decades ago and has led to the elimination of smallpox as a human health threat. This has been one of the greatest success stories in medicine. In the United States, the last confirmed case of smallpox occurred in 1949, and worldwide the last recorded case of naturally occurring smallpox occurred in Somalia in 1977. In 1980, the World Health Organization formally declared that smallpox had been eradicated. There are currently only two official laboratory stockpiles of the smallpox virus in the world – one housed at the Centers for Disease Control and Prevention in Atlanta and the other at a research facility in Russia.
The Problem
The smallpox virus is classified as a highest risk Category A bioterrorism agent for several reasons.
- It is highly contagious.
- It has a high mortality rate.
- There is no known cure for smallpox.
- The vaccine used to eradicate smallpox can lead to complications and death, especially among older persons.
- The general population lacks immunity to smallpox. Routine smallpox vaccination ended in the United States in 1972. Even people vaccinated before 1972 are considered susceptible to smallpox infection.
- It is considered likely that secret stockpiles of smallpox virus exist outside of the official WHO repositories, and the extent and locations of these stockpiles are unknown.
The Research
Investigators in the Department of Molecular Virology and Microbiology (MVM) have obtained funding from several sources to study immune responses to the smallpox virus and for vaccine development studies.
Dr. Innocent Mbawuike has investigated the vulnerability of the general population to smallpox. Discontinuation of the smallpox vaccination program in the early 1970s has reduced immunity to the variola virus in the general population. Because of declining immune response activity with age, the elderly population is considered to be particularly susceptible to smallpox infection, and this group is also thought to mount a less effective immune response to vaccination against smallpox than younger individuals. Dr. Mbawuike has studied the longevity of specific immune responses to the smallpox virus in the general U.S. population. A goal of his work has been to compare the immune responses against vaccinia virus (used as a vaccine) in representative young adult and elderly populations by examining antibody and cell-mediated (specifically CD8 + CTL memory) immune activity in these groups. He has also worked to identify easily detectable and quantifiable markers for effective immune responses against vaccinia that may be used as a surrogate of immunity to smallpox infection. In the event of a biological attack with smallpox, the results of Dr. Mbawuike’s work should provide guidance in decisions to revaccinate the public, particularly the elderly and other immunodeficient persons.
In another project, MVM researchers have applied a new technology to identify the vaccinia virus antigens that are responsible for the anti-smallpox humoral and cell-mediated immunity that confers protection against smallpox infection. The goal of this work has been to identify antigens that would be candidates for use in developing new smallpox vaccines. The technology is called the TAP (Transcriptionally Active PCR) Express TM system and is a tool that allows hundreds or thousands of antigen genes to be individually amplified in a transcriptionally active form so that their biological function or DNA vaccine immunological potency can be quickly analyzed. MVM researchers have been applying this technology to the production of all the proteins encoded by vaccinia virus to identify antigens that might be useful for the production of DNA vaccines to smallpox. This project has been funded by a Small Business Innovation Research subcontract from Gene Therapy Systems, Inc.
MVM investigators have also participated in smallpox vaccine trials. They have tested two different smallpox vaccines - the freeze-dried live virus commercial smallpox Dryvax vaccine (Wyeth-Lederle Vaccines) currently approved for use in the United States and the frozen smallpox vaccine (APSV) produced by Aventis Pasteur. Both vaccines were prepared more than 30 years ago. Approximately 500 subjects have participated to date in these studies at BCM. These trials demonstrated the safety and potency of the Dryvax and APSV vaccines. Importantly, these studies showed that the vaccines could remain potent following a 10-fold dilution, effectively increasing a 15 million dose stockpile to 150 million doses. This work has been conducted within the BCM Viral Respiratory Pathogens Research Unit (VRPRU, headed by Dr. Robert Couch) and the BCM Vaccine Trial Evaluation Unit (VTEU, headed by Dr. Wendy Keitel). These groups are also planning to evaluate a new generation of smallpox vaccines (Modified Vaccinia Ankara and LC16m8 ) that produce fewer adverse reactions.
Glossary
Learn more about some of the technical terms found on this page by visiting our glossary of terms.








 Credit
Credit