The Agents
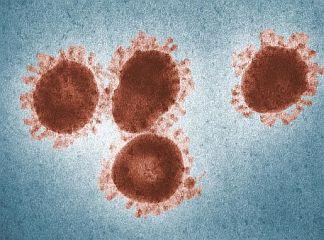
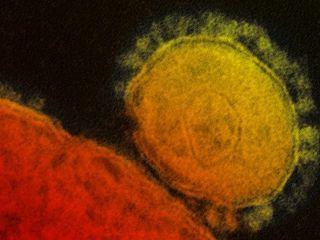
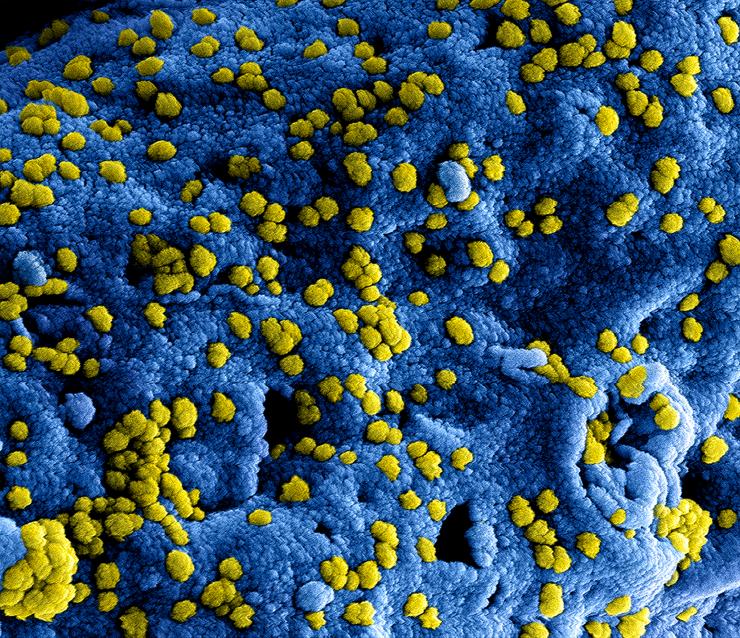
SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome) are serious infectious respiratory diseases that are caused by members of a class of viruses known as coronaviruses. The name coronavirus comes from the appearance of the virus under the microscope – it has a spiky or crown-like (corona) appearance. Both diseases can be fatal to humans.
SARS is caused by SARS-associated coronavirus (SARS-CoV), while MERS is caused by Middle East respiratory syndrome coronavirus (MERS-CoV).
SARS-CoV emerged about two decades ago in China and spread rapidly to other countries. Fortunately, it was successfully contained and no new cases have been reported since the initial outbreak. MERS-CoV emerged in 2012 in Saudi Arabia and circulated throughout the Middle East and was carried by travelers to other parts of the world including the United States, Europe, Africa, and Asia. The rate of new infections has since dropped significantly.
More recently, late in 2019, a third member of the coronavirus family capable of causing severe disease arose. The new virus, emerging in Wuhan, China, is called SARS-CoV-2 and is responsible for the disease known as COVID-19. SARS-CoV-2 spread quickly around the globe. Unlike SARS-CoV or MERS-CoV, SARS-CoV-2 was not able to be controlled, resulting in a worldwide pandemic.
SARS
SARS was first recognized in Guangdong Province, China in November of 2002. It then spread extremely rapidly to other regions within China, Hong Kong, Vietnam, Singapore, Taiwan, and Toronto, Canada in the early half of 2003.
SARS is characterized by severe, pneumonia-like symptoms which can be fatal. SARS-CoV was transmitted from person to person mainly through respiratory droplets produced when a person sneezes or coughs and through direct contact with a surface contaminated with infected respiratory droplets.
Altogether, more than 8,000 people were documented to have been infected with SARS-CoV and over 800 died.
The global scientific response to SARS was unprecedented. Within weeks after the respiratory disease was first reported, the agent that causes the disease was identified, diagnostic tests were developed, and the entire genome of the virus was sequenced.
Epidemiologists gathered evidence that the first people infected had had contact with wild game in the markets of Guangdong Province in China. It is likely that these individuals were infected through direct contact with infected animals, particularly palm civets, which harbored very closely related coronaviruses. The virus then is thought to have mutated to adapt to its human host, and consequently human-to-human transmission became more efficient, setting off the SARS epidemic.
Fortunately, the SARS outbreak was short-lived, and public health containment procedures and coordinated responses proved effective in preventing further spread of the disease.
Until recently, SARS-CoV was the only member of the coronavirus family known to cause death or severe respiratory disease in humans. The other previously known viruses in this group cause mild upper-respiratory infections in humans and are associated with respiratory, gastrointestinal, and neurologic diseases in animals.
One reason that SARS-CoV might have been more lethal than other coronaviruses is that it appears to interfere with an enzyme system in humans that is critical for regulating body fluid balance. Therefore, the virus could disrupt normal functioning of the lungs by blocking this enzyme system and allowing fluid to leak into the air sacs of the lungs, resulting in severe respiratory illness.
MERS
A new member of the coronavirus family, MERS-CoV, emerged in the fall of 2012 in the Arabian Peninsula. Although MERS-CoV is distinct from SARS-CoV, the disease caused by MERS is similar to SARS, in that it is characterized by a severe respiratory illness that can be fatal in humans. The mortality rate is about 35 percent, although this figure may be an overestimate as mild cases may not have been reported and included in the calculations. People with certain conditions, such as diabetes, renal failure, chronic lung disease, and immunocompromised persons, as well as the elderly, are considered to be at high risk of severe disease from MERS-CoV infection.
Like SARS, MERS is thought to have originated in bats, but MERS-CoV has been passed to dromedary camels, which serve as the primary source of human MERS-CoV infection. Once a person becomes infected with MERS-CoV, it can be spread to unprotected people who come into close contact such as healthcare workers and, less frequently, family members. However, the virus is not easily transmitted from person to person and there has been no sustained transmission of MERS-CoV.
The first and vast majority of cases of MERS have occurred in Saudi Arabia, although infections have been reported in other countries in the region. Travel-associated cases have been reported in countries in Europe, Asia, Africa, and North America. Two unrelated MERS-CoV infections were confirmed in the United States in May 2014 in healthcare providers who had worked in Saudi Arabia. In May 2015, the first reported case of human-to-human transmission of MERS-CoV outside the Middle East was reported in South Korea where a traveler from the Middle East transmitted the virus to an unusually large number of people through contact with healthcare workers, other patients, or family members.
As of May 2020, the total number of laboratory-confirmed MERS-CoV infection cases reported globally is over 2,500 with nearly 900 associated deaths. In total, 27 countries have reported cases since 2012, with 80% of these cases occurring in Saudi Arabia.
The Problem
At this time, there are no approved vaccines or specific treatments for SARS or MERS. However, several vaccines and treatments are in development.
Although there are currently no known human cases of SARS, it is still possible that another outbreak of SARS could occur. It is probable that SARS-CoV still lurks in an animal host in the wild, and human contact with this animal(s) could again spark a SARS epidemic. Scientists have reported that the Chinese horseshoe bat is likely to be the animal that is the hiding place of the SARS virus. Genetic analysis of the virus in bats showed that it is closely related to the human SARS virus, although it is still not clear how the SARS virus was transmitted from bats to humans.
MERS-CoV has continued to spread as recently as 2020, albeit at very low rates, but the potential for a further outbreak remains. The MERS outbreak in 2015 provided a warning of how easily “new” diseases can be transported to other continents and spread before the disease is recognized. After the virus arrived in South Korea, carried by a single traveler from the Middle East, the virus spread quickly through a hospital setting that did not take precautions against the unrecognized disease. In a little over a month, there were over 180 infections, including more than 30 deaths, before the man’s illness was identified as MERS and he and his contacts were isolated.
On a much smaller scale, this MERS outbreak foreshadowed the subsequent SARS-CoV-2 outbreak by exacting an economic and political toll within South Korea, as schools and businesses were shuttered, and people were fearful of visiting public places. This also illustrates the importance of remaining vigilant in order to quickly identify the agent of infection and enact immediate infection control measures, such as restricting contact with infected individuals.
The Research
Following the SARS outbreak of 2003, investigators in the Department of Molecular Virology and Microbiology at Baylor College of Medicine were awarded funds for development of a SARS-CoV research program (as an expansion of the existing Virus Respiratory Pathogens Research Unit in a contract arrangement between BCM and the National Institutes of Health). The program focused on the pathogenesis of SARS-CoV infection and disease, development of a virus-like particle vaccine for SARS, and clinical trials of a SARS vaccine.
Results of an evaluation of candidate vaccines against the SARS virus were published by Drs. Robert Atmar and Robert Couch, along with colleagues at The University of Texas Medical Branch in Galveston, Texas. A whole virus inactivated vaccine was tested in ferrets and nonhuman primates and a virus-like particle vaccine was tested in mice. The vaccines provided protection against infection. However, the vaccines caused damage to the lungs of mice infected with the virus, so it is unlikely that these vaccine candidates would be developed for use in humans.
In another approach, Texas Children's Center for Vaccine Development (Texas Children's CVD) co-led by Drs. Peter Hotez and Maria Elena Bottazzi of the Department of Pediatrics – Tropical Medicine, the Department of Molecular Virology and Microbiology, and the National School of Tropical Medicine at Baylor, received funding from the National Institute of Allergy and Infectious Diseases of the NIH to develop recombinant protein-based SARS and MERS vaccines.
Texas Children's CVD and their collaborators based their vaccine development on a segment of the SARS-CoV or MERS-CoV spike protein (a protein found on the outside of the virus that interacts with the host cell) known as the receptor binding domain (RBD). The idea is that the vaccines would stimulate neutralizing antibodies which would block the attachment of the virus to its receptor on the host cell, thus preventing infection by the coronaviruses. The researchers have shown that the RBD vaccine candidate does elicit a strong neutralizing antibody response and protects vaccinated animals against a challenge viral infection.
In order to optimize a potential vaccine, the investigators have made modifications to the RBD protein and tested the various candidates in mice. They have found an RBD construct that induced significantly stronger RBD-specific antibody responses and a higher level of neutralizing antibodies in immunized mice than the original version. The optimized vaccine candidates have now been used as the basis for the further development of other coronavirus vaccines.
Drs. Bottazzi and Hotez and their colleagues are applying the knowledge gained during their investigations of SARS and MERS vaccine candidates to the development of a vaccine against another coronavirus, SARS-CoV-2, the virus that causes COVID-19.
Coronavirus vaccines would be beneficial to help avert potential future emergent outbreaks, as well as for biodefense preparedness in the event of the deliberate release of a coronavirus as an agent of bioterrorism. The scientists continue to advocate for prioritized funding for coronavirus vaccine research.
Glossary
Learn more about some of the technical terms found on this page by visiting our glossary of terms.








 Credit
Credit