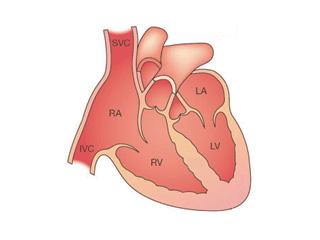
The heart is a powerful pump made of muscle (myocardium). It is divided into four chambers: the upper two atria are the receiving chambers and the lower two ventricles are the pumping chambers.
Dilated cardiomyopathy (DCM) causes the heart to become enlarged, particularly the left ventricle, and to function (squeeze and pump) poorly. As a result, the heart muscle becomes weak and thin and is unable to pump blood efficiently around the body. This causes fluid to build up in the lungs, which become congested, and results in a feeling of breathlessness. This condition is called "left heart failure." Often there is also right heart failure, which causes fluid to accumulate in the tissues and organs of the body, usually the legs and ankles, and the liver and abdomen.
Frequency of DCM
Congestive heart failure due to poor heart function is a serious disease and a major cause of death and disability in children and adults. DCM, which is the most common cause of CHF, occurs in at least 100,000 individuals in the United States, and approximately 15,000 new cases are identified each year, although many asymptomatic cases go unrecognized. DCM is the most common diagnosis leading to heart transplantation.
Causes of DCM
In the majority of cases of DCM, the cause is not identified and is termed "idiopathic DCM." However, a number of causes have now been identified in some patients and are termed "acquired" (due to virus infection, coronary artery disease, alcohol and drug abuse, or pregnancy, etc.) or "familial" (due to the inheritance of a defective gene).
Viral Infection
A number of viruses have been identified in the hearts of patients with DCM. In 1986, Coxsackievirus B was identified in the hearts of patients with either DCM or a condition called viral myocarditis, in which the heart muscle becomes inflamed. Subsequently, work performed by Dr. Jeffrey Towbin and the Muzzy lab has shown that another virus, called adenovirus, is also commonly a cause of these conditions.
Some researchers have suggested that when the virus infects the heart, it causes the body to react against the virus, much as the body's own defense system (immune system) normally attacks a virus infection, and this causes inflammation in the heart (myocarditis). Usually, the virus will be eliminated. However, in some patients, the immune system may not totally eliminate the virus, and in these patients, the heart muscle continues to be damaged by the virus, leading to DCM. In some people, the body's immune system attacks and damages the heart, even though the virus has been eliminated: this is termed "auto-immune disease." Investigations into the role of viruses in the development of myocarditis and DCM are ongoing in the Phoebe Willingham Muzzy Pediatric Molecular Cardiology Laboratory, while viral diagnosis is provided by the John Welsh Cardiovascular Diagnostic Laboratory.
Auto-Immune Disease
The body's own immune system is responsible for defending it against all foreign invaders, including viruses and bacteria. However, this system sometimes malfunctions and starts to attack the body's own tissues. This results in a so-called "auto-immune disease." It is thought that this process occurs in some cases of DCM.
Alcohol and Drugs
Excessive alcohol consumption is one of the most common causes of DCM. If an excessive alcohol intake is stopped before serious damage to the heart has occurred, then the heart can recover. In some cases, however, the damage is too great and DCM persists lifelong. Other chemicals, such as certain anti-cancer therapies, as well as recreational drugs, such as cocaine, have been reported to cause DCM.
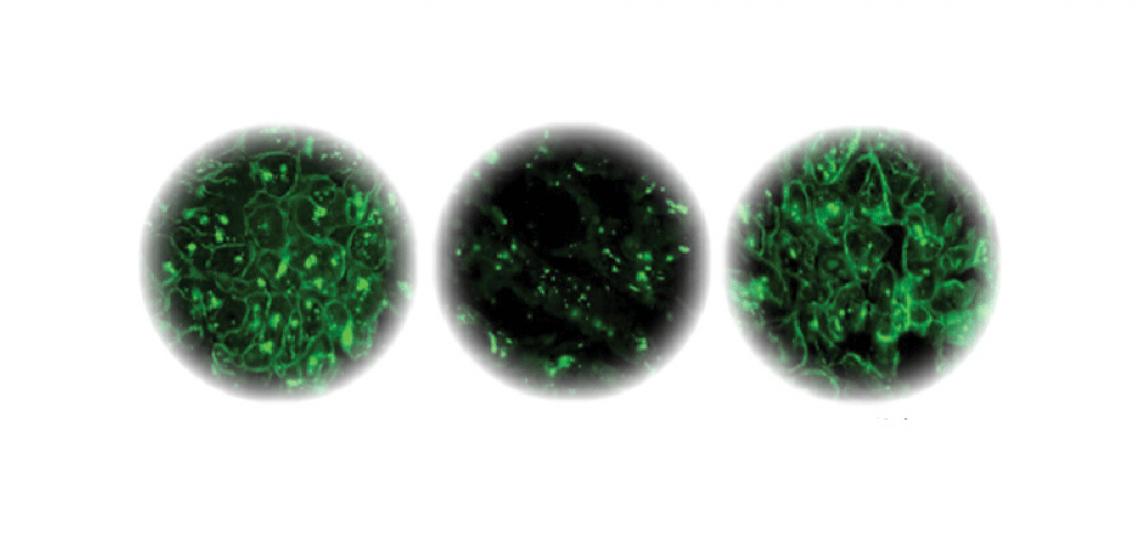
Left: Dystrophin staining of normal heart muscle. Center: Dystrophin staining of failing heart muscle. Right: Dystrophin staining of failing heart muscle after LVAD implantation.
Pregnancy
Although not common, women in mid to late pregnancy, or soon after delivery, can develop a form of DCM, termed "postpartum" or "peripartum cardiomyopathy." This occurs in approximately 1 in 10,000 pregnancies. It is possible that some of these women actually have DCM due to one of the other causes described here, which is exacerbated due to the extra demands placed on the heart during pregnancy. However, the cause in most cases is unknown. Postpartum cardiomyopathy usually resolves within eight weeks of delivery but may recur in subsequent pregnancies. Women who have not completely recovered are advised to avoid having further pregnancies.
Ischemic Cardiomyopathy
Common in adults, this form of DCM is uncommon in children. The usual cause of this disorder is coronary artery obstruction. In children, anomalous left coronary artery from the pulmonary artery or transplant coronary vasculopathy may cause a form of ischemic DCM.
Familial DCM
All hereditary information is transmitted through DNA, which encodes many genes (approximately 100,000) that are transcribed to make specific proteins. Inherited disorders due to a single abnormal gene are transmitted to offspring in a predictable fashion, termed Mendelian transmission. As a result of gene mutations, abnormal genes located on any of the 22 autosomal pairs or the two sex chromosomes may produce phenotypes inherited by simple patterns classified as autosomal (dominant or recessive) or X-linked, respectively. When different genes induce the same phenotype, it is referred to as genetic heterogeneity, and most diseases in humans exhibit genetic heterogeneity.
Families with multiple individuals who have DCM are likely to have a genetically inherited form, termed "familial DCM." However, not all affected individuals must have a clinically affected parent because, in all autosomal dominant diseases, a certain proportion of cases occur due to a new mutation (i.e., they are sporadic). The parent whose germ cells contain the new mutation will be clinically normal because the mutation affects only a single germ cell, but can transmit the disease-causing allele to their offspring.
Approximately 30-40 percent of patients with DCM have a familial form of disease. Autosomal dominant inheritance is the predominant pattern of transmission, but X-linked, autosomal recessive, and mitochondrial inheritances also occur. When it occurs, mitochondrial inheritance is seen most commonly in infantile forms of familial DCM, whereas X-linked and autosomal recessive forms are probably evenly mixed between childhood and adult forms of disease.
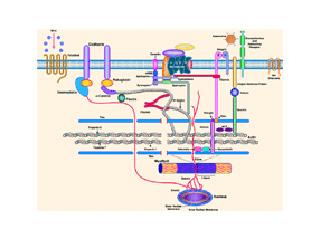
X-linked DCM was first described in 1987 as DCM occurring in males in the teen years and early twenties, with rapid progression from congestive heart failure to death or transplantation. Dr. Jeffrey Towbin and colleagues first identified the disease-causing gene as dystrophin. Dystrophin is a cytoskeletal protein that provides structural support to the muscle cell, as well as linking the contractile apparatus of the cell to the surface. At one end (N-terminus), dystrophin binds to actin, a key component of the cellular cytoskeleton as well as a member of the thin filament of the contractile apparatus. At the other end (C-terminus), dystrophin interacts with β-dystroglycan, and the dystrophin-associated glycoprotein complex, which includes α-dystroglycan, the sarcoglycan subcomplex (α, β, γ,δ,and ε sarcoglycan), syntrophins, and dystrobrevins. Mutations in a number of these proteins have been identified by the Muzzy lab, as well as other labs, in patients with DCM. For example, we have identified mutations in δ-sarcoglycan, α-dystrobrevin, lamin A/C, MLP, α-actinin, and ZASP. Genetic analysis of the lamin A/C gene is offered as a diagnostic test by the John Welsh Cardiovascular Diagnostic Laboratory.
Approximately 30-40 percent of patients with DCM have a familial form of disease. Autosomal dominant inheritance is the predominant pattern of transmission, but X-linked, autosomal recessive, and mitochondrial inheritances also occur. When it occurs, mitochondrial inheritance is seen most commonly in infantile forms of familial DCM, whereas X-linked and autosomal recessive forms are probably evenly mixed between childhood and adult forms of disease.
X-linked DCM was first described in 1987 as DCM occurring in males in the teen years and early twenties, with rapid progression from congestive heart failure to death or transplantation. Dr. Jeffrey Towbin and colleagues first identified the disease-causing gene as dystrophin. Dystrophin is a cytoskeletal protein that provides structural support to the muscle cell, as well as linking the contractile apparatus of the cell to the surface. At one end (N-terminus), dystrophin binds to actin, a key component of the cellular cytoskeleton as well as a member of the thin filament of the contractile apparatus. At the other end (C-terminus), dystrophin interacts with β-dystroglycan, and the dystrophin-associated glycoprotein complex, which includes α-dystroglycan, the sarcoglycan subcomplex (α, β, γ,δ,and ε sarcoglycan), syntrophins, and dystrobrevins. Mutations in a number of these proteins have been identified by the Muzzy lab, as well as other labs, in patients with DCM. For example, we have identified mutations in δ-sarcoglycan, α-dystrobrevin, lamin A/C, MLP, α-actinin, and ZASP. Genetic analysis of the lamin A/C gene is offered as a diagnostic test by the John Welsh Cardiovascular Diagnostic Laboratory.
Barth Syndrome
Another X-linked form of cardiomyopathy, Barth syndrome, was initially described as X-linked cardioskeletal myopathy with abnormal mitochondria and neutropenia (low white blood count). This disorder typically presents in male infants as congestive heart failure associated with neutropenia and 3-methylglutaconic aciduria.
Barth syndrome is caused by mutations in the gene, G4.5 (TAZ). However, mutations in G4.5 result in a wide clinical spectrum, which includes apparent classic DCM, hypertrophic DCM, endocardial fibroelastosis, or left ventricular noncompaction, with or without other features of Barth syndrome. The identification of G4.5 mutations in affected patients and their family members is currently performed by the John Welsh Cardiovascular Diagnostic Laboratory.
In the Phoebe Willingham Muzzy Pediatric Molecular Cardiology Laboratory, we are continuing to identify the causes of familial DCM by screening the identified DCM genes, as well as searching for novel genes.








