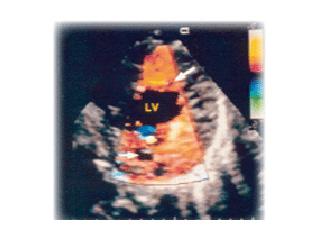
Left ventricular noncompaction is characterized by deep trabeculations in the LV endocardium in association with left ventricular hypertrophy, dilation or hypertrophy/dilation. Noncompaction of the ventricular myocardium is a rare congenital cardiomyopathy of children and adults resulting from arrested myocardial development during embryogenesis.
In some patients, the left ventricle is hypercontractile, whereas in others, systolic dysfunction predominates. Two forms of LVNC have been described, an isolated form and a form associated with congenital heart disease; in the latter case, this non-isolated LV noncompaction may be found in combination with septal defects, pulmonic stenosis or hypoplastic left ventricle. The two forms of LVNC are found in all age groups, although an infantile presentation appears to be most common; survival appears to be good.
In this disorder, echocardiography demonstrates a thin epicardium with extremely hypertrophied endocardium and prominent trabeculations with deep recesses. These features tend to be apically localized.
Frequency of LVNC
The incidence of LVNC is unknown, but considered rare. However, many cases likely are overlooked due to the often subtle echocardiographic changes being mistaken for dilated cardiomyopathy or hypertrophic cardiomyopathy.
Causes of LVNC
All hereditary information is transmitted through DNA, which encodes many genes (approximately 100,000) that are transcribed to make specific proteins. Inherited disorders caused by a single abnormal gene are transmitted to offspring in a predictable fashion, termed Mendelian transmission. As a result of gene mutations, abnormal genes located on any of the 22 autosomal pairs or the two sex chromosomes may produce phenotypes inherited by simple patterns classified as autosomal (dominant or recessive) or X-linked, respectively. When different genes induce the same phenotype, it is referred to as genetic heterogeneity, and most diseases in humans exhibit genetic heterogeneity.
Families with multiple individuals who have LVNC are likely to have a genetically inherited form. Not all affected individuals, however, must have an affected parent because, in all autosomal dominant diseases, a certain proportion of cases occur due to a new mutation (i.e., they are sporadic). The parent whose germ cells contain the new mutation will be clinically normal because the mutation affects only a single germ cell, but the disease-causing allele can be transmitted to their offspring.
In isolated LVNC, familial recurrence is high and found in approximately 40 percent of patients. The inheritance patterns of LVNC include X-linked inheritance in males with isolated LVNC and an autosomal dominant pattern in some isolated and non-isolated cases. In the X-linked form, mutations in the gene G4.5 (TAZ), which encodes tafazzin, were initially identified by Bleyl and colleagues. This is the same gene previously identified by Bione and colleagues as causative of Barth syndrome; some patients with LVNC also have clinical features of Barth syndrome.
Recently, we identified a gene for NLVNC in a family with broad clinical features ranging from apparent isolated LVNC, to LVNC with septal defects alone, to LVNC associated with hypoplastic left ventricle. This gene encodes alpha-dystrobrevin, which is a dystrophin-associated protein mapped to chromosome 18q12. This DGC protein helps to maintain structural integrity of the muscle membrane, but also has signaling functions via the nitric oxide synthase pathway and is also a substrate for tyrosine kinases. Mutant mice develop cardiomyopathy when this gene is knocked out, supporting it as the cause of ventricular dysfunction. Again, skeletal myopathy is a feature to be noted. It is possible that the cardiac muscle abnormality is caused by disturbance of structural integrity and the congenital heart disease is due to perturbation in these signaling pathways in humans.
In the John Welsh Cardiovascular Diagnostic Laboratory, we offer analysis of the G4.5 (TAZ) gene for genetic mutations.








